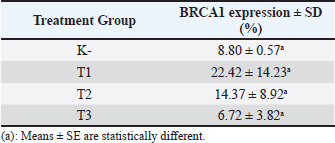| Research Article | ||
Open Vet. J.. 2024; 14(10): 2678-2686 Open Veterinary Journal, (2024), Vol. 14(10): 2678–2686 Research Article Black soybean extract inhibits rat mammary carcinogenesis through BRCA1 and TNF-α expression: In silico and in vivo studyDyah Ayu OA Pratama1, Annesia Fernanda2, Ricadonna Raissa3*, Fajar Shodiq Permata1 and Muhammad Luqman Nordin4,51Department of Pathology, Faculty of Veterinary Medicine, Universitas Brawijaya, Malang, East Java, 65151, Indonesia 2Undergraduate Student, Faculty of Veterinary Medicine, Universitas Brawijaya, Malang, East Java, 65151, Indonesia 3Departement of Pharmacology, Faculty of Veterinary Medicine, Universitas Brawijaya, Malang, East Java, 65151, Indonesia 4Department of Veterinary Clinical Studies, Faculty of Veterinary Medicine, Universiti Malaysia Kelantan, Kota Bharu, Kelantan, 16100, Malaysia 5Animal and Wildlife Research Group, Faculty of Earth Science, Universiti Malaysia Kelantan, Jeli Campus, Jeli, Kelantan, 17600, Malaysia *Corresponding Author: Ricadonna Raissa. Department of Pharmacology, Faculty of Veterinary Medicine, Universitas Brawijaya, Malang, Indonesia. Email: ricadonnaraissa [at] ub.ac.id Submitted: 14/04/2024 Accepted: 03/09/2024 Published: 31/10/2024 © 2024 Open Veterinary Journal
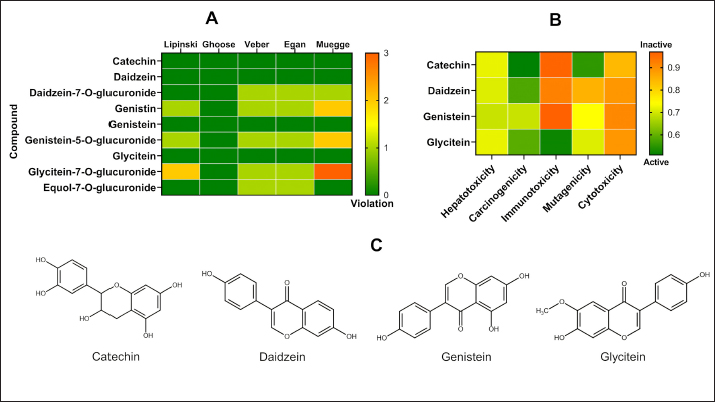
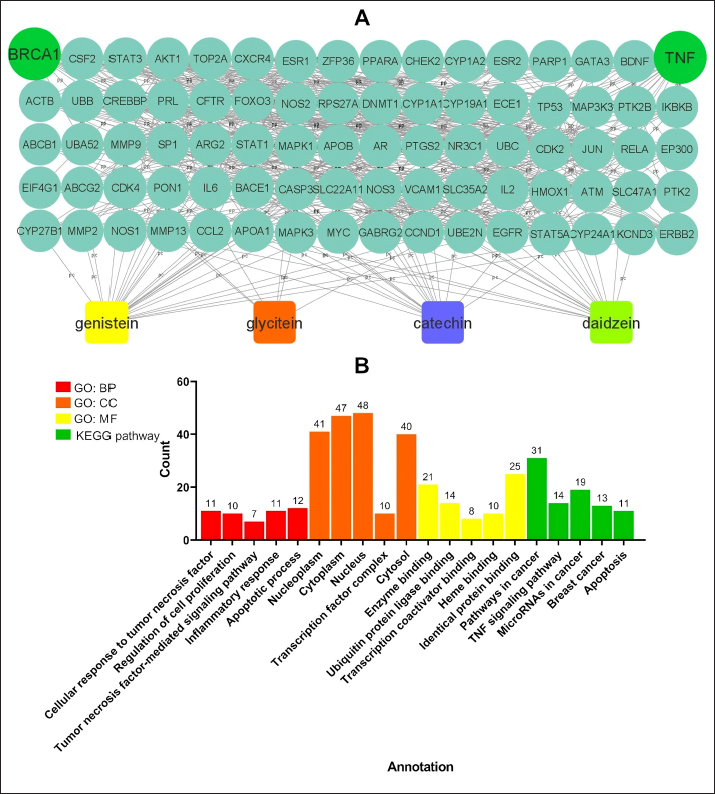
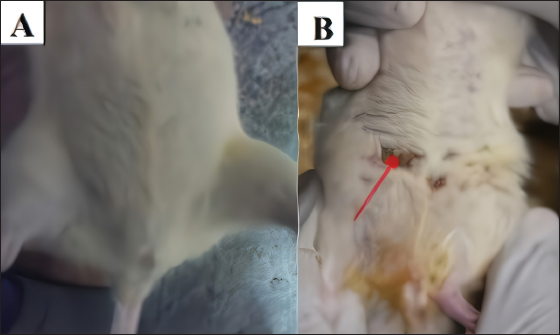
AbstractBackground: Mammary gland carcinoma is a malignant type of cancer that occurs in mammae tissue. Dimethylbenzene (α) anthracene (DMBA) is a carcinogenic agent that causes mammary cancer by damaging cellular DNA. Flavonoids found in the black soybean (Glycine max L. Merr) exhibit anti-carcinogenic effects. Aim: This study evaluated the anticarcinogenic effects of black soybean extract. Methods: The activity of flavonoid compounds in black soybean was determined in silico. Five groups of rats, four in each group, were established, consisting of a negative control, a positive control, and three treatment groups. Treatment included black soybean extract administration (i.e., T1=200, T2=400, and T3=800 mg of black soybean extract/kg body weight for 10 days). The observed parameters included the immunohistochemical analysis of Breast Cancer 1(BRCA1) and TNF-α. Results: Based on an in silico study, compounds from black soybeans are non-toxic. Functional annotation analysis revealed that most of the target proteins have a role in biological processes associated with cancer development. An in vivo analysis using an animal mammae cancer model indicated that black soybean extracts inhibited mammae cancer progression by attenuating TNF-α and BRCA1 expression. Conclusion: The most effective dosage of black soybean extract was 200 mg/kg body weight. An increase in BRCA1 and TNF-α expression may be related to the effects of catechin, daidzein, genistein, and glycitein, which are present in black soybeans. Keywords: Mammary cancer, Dimethylbenzene (α) anthracene, Breast cancer-1, Tumor necrosis factor alpha, Immunohistochemistry. IntroductionMammary gland carcinoma is a common cancer that occurs in female animals, such as dogs, cats, and horses, with dogs being the most common (Baba and Câtoi, 2007). The incidence of mammary gland carcinoma in female dogs and cats is approximately 41%–53% and 86%, respectively (Todorova, 2009). Many studies have been done to determine the underlying molecular mechanism of mammary gland carcinoma. DMBA is a carcinogenic substance that is widely used for in vivo studies related to cancer, such as mammary carcinomas (Kerdelhué et al., 2016). DMBA is metabolized to an epoxidedihydrodiol form of the epoxide dihydrodiol compound. The active compound forms a covalent bond with DNA, which results in DNA damage. The active metabolism of DMBA also increases reactive oxygen species (ROS) production in premalignant cells (Mori et al., 2022). The study of protein expression in cancer cells may provide information regarding treatment efficacy. Well-known proteins expressed in breast cancer are Breast Cancer 1 (BRCA1) and tumor necrosis factor alpha (TNF-α). BRCA1 is a tumor suppressor gene that stimulates the formation of proteins that repair DNA damage. The protein prevents cells from synthesizing and dividing rapidly. DMBA-induced DNA damage results in genetic mutations that disrupt BRCA1 function and promote carcinogenesis (Mateo et al., 2022). TNF-α is overexpressed in patients with metastatic breast cancer and induces apoptosis (Montfort et al., 2019). Indigenous herbal plants have enormous potential for treating cancer and should be a focus of drug development. Flavonoids are ubiquitous polyphenolic compounds found in plants. They exhibit anticancer effects by modulating ROS-scavenging enzyme activity, inducing cell cycle, promoting apoptosis, autophagy, invasiveness, and suppressing cancer cell proliferation (Montfort et al., 2019). Black soybeans have the potential to be anti-carcinogenic because they are one of the main dietary sources of flavonoids, such as catechin, daidzein, genistin, genistein, and glycitein (Kopustinskiene et al., 2020). Moreover, black soybeans have higher antioxidant activity compared with yellow soybeans (Hasim et al., 2015; Choi et al., 2020). Nurrahman (2015) found that black soybean contains 0.65 ± 0.07 mg/g of genistein, which is higher compared with that of yellow soybeans (0.40 ± 0.01 mg/g). Black soybean exhibits the highest antioxidant activity compared with other soybean types; thus, they have the potential to be an effective treatment for carcinoma mammae (Nurrahman, 2015). In this study, we evaluated the anti-carcinogenic effects of a black soybean extract in rat carcinoma mammae and BRCA1 and TNF-α expression in silico and in vivo. Materials and MethodsIn silico studyDrug likeness and toxicity screening The bioactive compounds contained in black soybeans were obtained from previous reports. Canonical SMILES of these compounds were obtained from the PubChem database. Drug-likeness screening was done using the SWISS ADME web server (www.swissadme.ch) using Lipinski, Egan, Veber, Muege, and Ghose parameters. Compounds that passed the drug-likeness screening were subject to toxicity analysis using the ProTox II web server (https://tox-new.charite.de/protox_II). Compounds with an inactive probability above 0.5 were considered non-toxic. Compounds that passed the selection criteria were subject to target protein prediction and the PPI construction stage. Protein target prediction and PPI construction Target protein prediction for each compound was conducted using the STITCH web server (http://stitch.embl.de/cgi) with a cut-off of 0.4 and a maximum search of 50 proteins. PPI construction was done using Cytoscape 3.7.2 by merging all target proteins. PPI is displayed in the form of a Grid Layout. Functional annotation analysis Functional annotation analysis was conducted using the Database for Annotation, Visualization, and Integrated Discovery (https://david.ncifcrf.gov/tools.jsp). Gene Ontology and KEGG pathway databases were also used. The analysis focused on biological activity and cellular pathways associated with cancer progression. In vivo studyDMBA solution and black soybean extract preparation DMBA (7,12-dimethylbenz (α)anthracene) was dissolved in corn oil and normal saline at a ratio of 3:1. This mixture was then poured into a sterile 50 ml conical tube and homogenized using a vortex mixer. Black soybeans (Glycine max L. Merr.) were cold-macerated by adding 70% ethanol and filtered. The supernatant was concentrated with a rotary evaporator (IKA® HB 10 digital, Germany). The extraction was done in UPT Materia Medica, Batu, Malang. In vivo study design and carcinoma mammae animal model Twenty female Sprague–Dawley rats (Rattus norvegicus), 6–8 weeks of age, and 160–180 mg body weight were obtained. The rats were caged under controlled conditions with a temperature of 26°C–29°C and a 12 hours light/dark cycle. Feeding and husbandry were performed under standard practice. The rats were treated with DMBA through subcutaneous intramammary injection on the right and left side every 48 hours for a total of 10 times. The injection volume was 1 ml at a dose of 10 mg/kg body weight. The rats were divided into five groups (i.e., negative control (K-) and three treatment groups). Treatment included three different dosages of black soybean extract administration (i.e., T1=200, T2=400, and T3=800 mg of black soybean extract/kg body weight using a gastric tube for 10 days). Immunohistochemistry was used to assess the expression of BRCA1 and TNF-α. Physical observations were conducted on the number and size of nodules formed, body weight, and physiological conditions. Euthanasia using cervical dislocation was performed after black soybean extract administration and necropsies were immediately performed. Mammary glands were collected in a formalin buffer solution of 10% and embedded in paraffin. Immunohistochemistry Paraffin-embedded mammary tissues were cut into 4–5 µm sections. After soaking in xylol for the deparaffinized and hydration process, the tissues were washed three times with PBS (pH 7.4), soaked in hydrogen peroxide for 20 minutes, and washed three times with PBS. The tissues were treated with protein block (1% BSA) for 10–30 minutes at room temperature and washed three times with PBS. Primary antibodies against BRCA1 (Thermo Fisher, Cat #PA5-88149) and TNF-alpha (Santa Cruz, Cat#sc-52746) were added and incubated at room temperature for 1 hour and washed three times with PBS. A secondary antibody was added, incubated at room temperature for 1 hour, and washed with PBS. Chromogen DAB (3,3-diaminobenzidine tetrahydrochloride) was incubated for 10–20 minutes at a temperature 20°C–25°C and washed with distilled water. The tissues were counterstained with hematoxylin, incubated for 5 minutes at room temperature, washed with aquadest, and mounted with entellan. Observations were conducted using a microscope (Optilab) at 400 × and 1,000 × magnification. Under a light microscope, the expression of BRCA1 and TNF-α proteins displayed a brown color, whereas those that did not express the proteins were purple/blue. Data analysis The data for the percent area expression of BRCA1 and TNF-α were analyzed using a one-way ANOVA (α=0.05) followed by a 5% Tukey test (<0.05) using SPSS Statistic 25 software. Ethical approval The animal model experimental protocols were approved by the Ethics Committee of the Institut Biosains, Universitas Brawijaya, Malang (protocol number 124-KEP-UB-2021). Results In silico studyScreening for drug-likeness and toxicity Drug-likeness selection can identify compounds that have drug-like characteristics. Of the nine compounds evaluated, four had drug-like characteristics because they did not violate the five drug-likeness parameters. These four compounds, which included catechin, daidzein, genistein, and glycitein (Fig. 1A), were selected for toxicity studies. Toxicity selection identifies nontoxic compounds based on hepatotoxicity, carcinogenicity, immunotoxicity, mutagenicity, and cytotoxicity parameters. The results indicated that the four active compounds were not toxic through oral administration as indicated by their inactivity probability values >0.5 (Fig. 1B). The structures of these compounds are shown in Figure 1C. Protein target and PPI network Protein target prediction based on a PPI network revealed that each active compound has protein targets associated with BRCA1 and TNF (Fig. 2A). In addition, the four compounds may also target other proteins associated with Breast Cancer progressions, including AKT1, CCND1, EGFR, CDK2, and MYC. Functional annotation revealed that most of the target proteins have a role in biological processes related to cancer development. Based on the Gene Ontology database, particularly for Biological Processes, 7–12 target proteins are associated with cancer development. The KEGG pathway database indicates that 11–31 proteins have roles related to cancer and inflammation (Fig. 2B). In vivo studyCancer nodule growth observation On the 12th day post-infection, the positive group had nodules or lumps on the abdomen (Fig. 3). Carcinoma mammae may be diagnosed by the presence of a lump in the area around the mammary gland, which is characterized by a solid rigid, and immobile mass. Palpation revealed a lump of solid mass that was hard and irregular in the mammae region, which could not be moved and appeared immobile. This indicated that a cancerous mass had formed.
Fig. 1. Screening of drug-likeness and toxicity for the compounds contained in black soybeans. A) Screening for drug-likeness, B) Toxicity screening, and C) 2D structure of compounds that passed the screening.
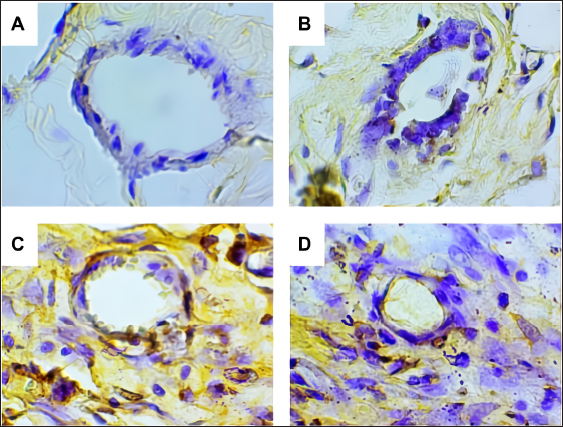
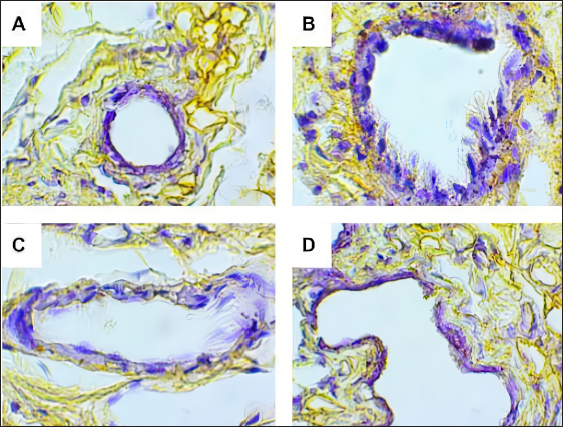
Fig. 2. Predicted protein targets and functional annotation. A) Predicted target proteins in the form of a PPI network. B) Functional annotation based on the GO and KEGG pathway databases. (BP: Biological process, CC: Cellular Component, MF: Molecular Function). Immunohistochemistry examination of BRCA1 expression Immunohistochemistry was used to count the number of cells expressing the BRCA1 gene. BRCA1 protein expression was detected by a brown color distributed in the nucleus of the acinar epithelial cells of the mammary glands (Fig. 4). Figure 4A shows a single layer of flat epithelial cells with some BRCA1 expression in the negative control group. Thickening of the epithelial cells resulting from inflammatory cell proliferation occurred; however, no BRCA1 expression was evident in the negative control group (Fig. 4A). In the treatment groups, there was a reduction in the thickening of the epithelium from T1 through T3. In T3, the single layer of flat epithelial cells was almost the same thickness as the epithelium of the negative control (Fig. 4B–D). Groups 1, 2, and 3 were treated with black bean extract at 200, 400, and 800 mg/kg body weight, respectively. All treatments had a therapeutic effect in the mammary cancer rat model. A decrease in BRCA1 expression was observed in each group, and a higher therapeutic dosage dose was associated with a concomitant reduction in BRCA1 expression (Table 1).
Fig. 3. The abdomen of rats on the 12th day observation. The negative group (A) does not show a nodule. The positive group (B) has nodules (red arrow).
Fig. 4. BRCA1 expression in the acinar cell nucleus of the rat mammary organ. A: Negative Control (K−); B: Group T1 dosage of 200 mg/kg body weight; C: Group T2 dosage of 400 mg/kg body weight; D; Group T3 dosage of 800 mg/kg body weight. Note: The black arrows (↑) indicate BRCA1 expression at 1,000 × magnification. An increase in BRCA1 expression in the mammary glands of the treatment group was observed following DMBA administration. The metabolite, DMBA-3,4-diol-1,2-epoxide, binds covalently to active cellular DNA to form DNA adducts, which results in mutations. DNA mutations disrupt cell proliferation and differentiation and promote the rapid growth of mammary cancer cells. In the treated groups, a dose of 200, 400, and 800 mg/kgBW extract had a therapeutic effect on mammary carcinogenesis in the rats. This was evident by a decrease in BRCA1 expression in each group. The higher the therapeutic dosage, the greater the reduction in BRCA1 expression. Table 1. The expression of BRCA1 in the Mammary glands of black soybean extract-treated rats.
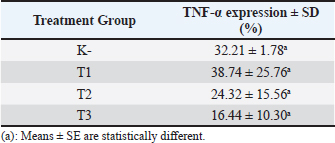
Immunohistochemistry of TNF- expression TNF-α is distributed in the cytoplasm of acinar or acini epithelial cells of the mammary gland as indicated by the brown color resulting from the binding of the antibody to its specific antigen (Fig. 5). The negative control showed a single layer of flat epithelial cells with little expression of TNF-α (Fig. 5A). The results indicated that the T1 group had reduced thickening of the epithelium (Fig. 5B). The T2 and T3 groups showed a single layer of flat epithelial cells that were similar in thickness to the negative control group (Fig. 5C, D). Based on the average expression of TNF-α, all black soybean extract treatments had a therapeutic effect in the mammary cancer rat model as evidenced by a decrease in TNF-α expression in each group (Table 2). The higher the therapeutic dosage, the greater the reduction of TNF-α expression (Table 2).
Fig. 5. Expression of TNF-α in the cytoplasm of epithelial cells. A: Negative Control (K−); B: Group T1 dosage of 200 mg/kg body weight; C: Group T2 dosage of 400 mg/kg body weight; D: Group T3 dosage of 800 mg/kg body weight. Note: The black arrows (↑) indicate TNF-α expression at 1,000 × magnification. Table 2. The expression of TNF-α in the mammary glands of black soybean extract-treated rats.
A statistical analysis was done by Tukey’s 5% post-hoc test to determine the significance of the observed differences. There was a difference in TNF-α expression in the T1, T2, and T3 treatment groups; however, it was not significant (p > 0.05). TNF-α expression in the T1, T2, and T3 groups was significantly different compared with that in the negative control (K-) group (p < 0.05). This indicates that the treatment (T1, T2, and T3) resulted in a therapeutic effect on breast cancer because a decrease in TNF-α expression was observed. DiscussionDirect administration of DMBA to the mammary gland causes interactions with DNA that result in the creation of DNA adducts and ultimately damage to DNA. This DNA damage has the potential to cause mutations in the mammary gland cells, which would impair normal physiological functions, subsequently inactivate tumor suppressor genes, and may result in uncontrolled cell development, which is a characteristic of carcinogenesis. The genes inactivation results in constant cell proliferation to occur (Ignasius, 2020). The nodules formed around the treated rat mammary glands were caused by an increase in cell proliferation from active metabolites of DMBA that were injected subcutaneously. DMBA-3,4-diol-1,2-epoxide (a metabolite of DMBA) can bind covalently with active cellular DNA to form DNA adducts and subsequently cause DNA mutations. DNA mutations disrupt cell proliferation and cell differentiation, and induce the rapid growth of mammary cancer cells (Zhang et al., 2018). Cancer is a disease characterized by abnormal cell proliferation caused by mutations, deletions, and a number of other factors that change the processes and signaling channels (Dibha et al., 2022). Studying the mechanisms behind DMBA-induced DNA damage and mammary carcinogenesis is essential for understanding the pathophysiology of mammary cancer and identifying potential therapeutic interventions. Researchers focus on exploring the cellular responses to DMBA exposure, including DNA repair mechanisms, cell cycle regulation, and signaling pathways involved in tumor development. Overall, delving deeper into the effects of DMBA on the mammary gland provides valuable insights into the molecular basis of mammary cancer initiation and progression, paving the way for innovative strategies in cancer prevention and treatment. Black soybeans have a high flavonoid content, including catechin, daidzein, genistein, and glycitein (Hasim et al., 2015; Kopustinskiene et al., 2020). Drug-likeness and toxicity screening revealed that catechin, daidzein, genistein, and glycitein were the most potent compounds in black soybeans. Flavonoid compounds found in black soybeans are known for their potent anticancer and anti-inflammatory properties. These compounds have been shown to inhibit the growth of cancer cells, induce cancer cell apoptosis (programmed cell death), and prevent the formation of new blood vessels that supply tumors (anti-angiogenesis). Flavonoids may also help to prevent DNA damage, reduce inflammation that can promote cancer growth, and modulate signal transduction pathways involved in cancer cell survival and proliferation. These four compounds also target proteins related to BRCA1 and TNF-α protein activation. Therefore, the anticancer and anti-inflammatory activities of black soybean may be related to the activity of these four compounds. Previous studies have indicated that catechins exhibit anticancer and anti-inflammatory activity in various cancer cell lines (Ezzat et al., 2019). Other studies have found that genistein and daidzein have activity in prostate cancer (Hämäläinen et al., 2007; Adjakly et al., 2013; Chen et al., 2019). Glycitein also exerts anticancer and anti-inflammatory activity. Previous studies have demonstrated that glycitein induces apoptosis and cell cycle arrest in human gastric and lung cancer cells (Deng and Scott 2000; Alhmoud et al., 2020). In the present study, the expression of BRCA1 and TNF-α in the treatment groups was observed. Thompson et al. reported that the induction of BRCA1 expression induced apoptosis or reduced tumor cell growth, which suggests an alternative therapeutic approach that may be tumor-specific (Thompson et al., 1995). This is consistent with our findings of increased BRCA1 expression in rats treated with black soybean extracts. Conversely, TNF-α is a cytokine produced by immune cells that can suppress tumor cell proliferation (Wang and Lin 2008; Montfort et al., 2019). TNF-α signaling induces apoptosis in various cell types. In the present study, we found that TNF-α expression was increased in the treated groups. This indicates that black soybean extract has the potential to reduce tumor growth. The optimal dosage of black soybean extract was 200 mg/kg body weight. A dosage of 400 and 800 mg/kg body weight did not result in higher expression of BRCA1 and TNF-α compared with that in rats treated with 200 mg/kg body weight. The results of this in silico study suggest that catechin, daidzein, genistein, and glycitein play an important role in increasing the expression of BRCA1 and TNF-α in rat models of breast cancer and preventing tumor growth. In addition to the in vivo study, increased BRCA1 and TNF-α expression was observed in the treated groups. Plant-derived flavonoids, such as black soybean, are effective when their bioavailability is increased, which enhances their value as a treatment for cancer. The consumption of dietary flavonoids may be recommended to prevent and control a variety of diseases. ConclusionThe administration of black soybean extract to DMBA-induced mammary cancer model rats reduced the expression of BRCA1 and TNF-α, with the greatest effect observed at 200 mg black soybean extract/kg body weight. The active compounds in the black soybean extract that reduced BRCA1 and TNF-α expression include catechin, daidzein, genistein, and glycitein. AcknowledgmentsThe authors would like to thank the Faculty of Veterinary Medicine, Universitas Brawijaya, for awarding the research grant. The authors also thank the Laboratory of Veterinary Pathology, Faculty of Veterinary Medicine, Universitas Brawijaya, for facilitating this research project. Conflict of interestThe authors have no conflicts of interest regarding this study. FundingThis work was supported by the DPP/SPP Research Grant from the Faculty of Veterinary Medicine, Universitas Brawijaya (grant number 30/UN10.F13.06/PN/2021) to Dyah Ayu Oktavianie Ardhiana Pratama. Authors’ contributionsConceptualization, DAOP, RR; Methodology, DAOP, RR, FSP; Validation, RR, MLN, FSP; Formal analysis, AF, RR, FSP; Investigation, RR, MLN, AF; Resources, DAOP, FSP; Data curation, DAOP, RR, AF, FSP, MLN; Writing-original draft preparation, DAOP, RR, AF, FSP, MLN; Writing-review editing, RR, MLN; Visualization, RR, AF; Supervision, DAOP, FSP; Project administration, AF; Funding acquisition, DAOP. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAdjakly, M., Ngollo, M., Boiteux, J.P., Bignon, Y.J., Guy, L. and Bernard-Gallon, D. 2013. Genistein and daidzein: different molecular effects on prostate cancer. Anticancer Res. 33, 39–44. Alhmoud, J.F., Woolley, J.F., Al-Moustafa, A.E. and Malki, M.I. 2020. DNA damage/repair management in cancers. Cancers (Basel) 12, 1050. Baba, A.I. and Câtoi, C. 2007. Comparative oncology. Bucharest, Romania: The Publishing House of the Romanian Academy. Chen, Y., Guo, S., Jiang, K., Wang, Y., Yang, M. and Guo, M. 2019. Glycitin alleviates lipopolysaccharide-induced acute lung injury via inhibiting NF-κB and MAPKs pathway activation in mice. Int. Immunopharmacol. 75, 105749. Choi, Y.M., Yoon, H., Lee, S., Ko, H.C., Shin, M.J., Lee, M.C., Hur, O.S., Ro, N.Y. and Desta, K.T. 2020. Isoflavones, anthocyanins, phenolic content, and antioxidant activities of black soybeans (Glycine max (L.) Merrill) as affected by seed weight. Sci. Rep. 10, 19960. Deng, C.X. and Scott, F. 2000. Role of the tumor suppressor gene Brca1 in genetic stability and mammary gland tumor formation. Oncogene 19, 1059–1064. Dibha, A.F., Wahyuningsih, S., Ansori, A.N.M., Kharisma, V.D., Widyananda, M.H., Parikesit, A.A., Sibero, M.T., Probojati, R.T., Murtadlo, A.A.A., Trinugroho, J.P., Sucipto, T.H., Turista, D.D.R., Rosadi, I., Ullah, M.E., Jakhmola, V. and Zainul, R. 2022. Utilization of secondary metabolites in algae kappaphycus alvarezii as a breast cancer drug with a computational method. Pharmacogn J. 14(3), 536–543. Hämäläinen, M., Nieminen, R., Vuorela, P., Heinonen, M. and Moilanen, E. 2007. Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm. 2007, 45673. Hasim, A.P., Falah, S. and Faridah, D.N. 2015. Bacillus subtilis natto fermentation to improve aglycone isoflavones content of black soybean varieties detam 2. Int. Food Res. J. 22, 2558–2564. Ignasius, R.A.P.J. 2020. Nuts and seeds in health and disease prevention, 2nd ed. Cambridge, UK: Academic Press, pp: 147–159. Kerdelhué, B., Forest, C. and Coumoul, X. 2016. Dimethyl-Benz(a)anthracene: a mammary carcinogen and a neuroendocrine disruptor. Biochem. Open 3, 49–55. Kopustinskiene, D.M., Jakstas, V., Savickas, A. and Bernatoniene, J. 2020. Flavonoids as anticancer agents. Nutrients 12, 457. Mateo, F., He, Z., Mei, L., de Garibay, G.R., Herranz, C., García, N., Lorentzian, A., Baiges, A., Blommaert, E., Gómez, A., Mirallas, O., Garrido-Utrilla, A., Palomero, L., Espín, R., Extremera, A.I., Soler-Monsó, M.T., Petit, A., Li, R., Brunet, J., Chen, K., Tan, S., Eaves, C.J., McCloskey, C., Hakem, R., Khokha, R., Lange, P.F., Lázaro, C., Maxwell, C.A. and Pujana, M.A. 2022. Modification of BRCA1-associated breast cancer risk by HMMR overexpression. Nat. Commun. 13(1), 1895. Montfort, A., Colacios, C., Levade, T., Andrieu-Abadie, N., Meyer, N. and Ségui, B. 2019. The TNF paradox in cancer progression and immunotherapy. Front. Immunol. 10, 1818. Mori, Y., Kobayashi, H., Fujita, Y., Yatagawa, M., Kato, S., Kawanishi, S., Murata, M. and Oikawa, S. 2022. Mechanism of reactive oxygen species generation and oxidative DNA damage induced by acrylohydroxamic acid, a putative metabolite of acrylamide. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 873, 503420. Nurrahman, N. 2015. Evaluasi komposisi zat gizi dan senyawa antioksidan kedelai hitam dan kedelai kuning. J. Apl. Teknol. Pangan. 4(3), 89–93. Thompson, M.E., Jensen, R.A., Obermiller, P.S., Page, D.L. and Holt, J.T. 1995. Decreased expression of BRCA1 accelerates growth and is often present during sporadic breast cancer progression. Nat. Genet. 9, 444–450. Todorova I. 2009. Prevalence and etiology of the most common malignant tumours in dogs and cats. Bulg. J. Vet. Med. 9, 85–98. Wang, X. and Lin, Y. 2008. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol. Sin. 29, 1275–1288. Zhang, J., Guo, Q., Wei, M., Bai, J., Huang, J., Liu, Y., Su, Z. and Qiu, X. 2018. Metabolite identification and pharmacokinetic profiling of isoflavones from black soybean in rats using ultrahigh-performance liquid chromatography with linear-ion-trap–orbitrap and triple-quadrupole tandem mass spectrometry. J. Agric. Food Chem. 66, 12941–12952. | ||
| How to Cite this Article |
| Pubmed Style Pratama DAO, Fernanda A, Raissa R, Permata FS, Nordin ML. Black soybean extract inhibits rat mammary carcinogenesis through BRCA1 and TNF-α expression: In silico and in vivo study. Open Vet. J.. 2024; 14(10): 2678-2686. doi:10.5455/OVJ.2024.v14.i10.17 Web Style Pratama DAO, Fernanda A, Raissa R, Permata FS, Nordin ML. Black soybean extract inhibits rat mammary carcinogenesis through BRCA1 and TNF-α expression: In silico and in vivo study. https://www.openveterinaryjournal.com/?mno=183530 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i10.17 AMA (American Medical Association) Style Pratama DAO, Fernanda A, Raissa R, Permata FS, Nordin ML. Black soybean extract inhibits rat mammary carcinogenesis through BRCA1 and TNF-α expression: In silico and in vivo study. Open Vet. J.. 2024; 14(10): 2678-2686. doi:10.5455/OVJ.2024.v14.i10.17 Vancouver/ICMJE Style Pratama DAO, Fernanda A, Raissa R, Permata FS, Nordin ML. Black soybean extract inhibits rat mammary carcinogenesis through BRCA1 and TNF-α expression: In silico and in vivo study. Open Vet. J.. (2024), [cited January 25, 2026]; 14(10): 2678-2686. doi:10.5455/OVJ.2024.v14.i10.17 Harvard Style Pratama, D. A. O., Fernanda, . A., Raissa, . R., Permata, . F. S. & Nordin, . M. L. (2024) Black soybean extract inhibits rat mammary carcinogenesis through BRCA1 and TNF-α expression: In silico and in vivo study. Open Vet. J., 14 (10), 2678-2686. doi:10.5455/OVJ.2024.v14.i10.17 Turabian Style Pratama, Dyah Ayu Oa, Annesia Fernanda, Ricadonna Raissa, Fajar Shodiq Permata, and Muhammad Luqman Nordin. 2024. Black soybean extract inhibits rat mammary carcinogenesis through BRCA1 and TNF-α expression: In silico and in vivo study. Open Veterinary Journal, 14 (10), 2678-2686. doi:10.5455/OVJ.2024.v14.i10.17 Chicago Style Pratama, Dyah Ayu Oa, Annesia Fernanda, Ricadonna Raissa, Fajar Shodiq Permata, and Muhammad Luqman Nordin. "Black soybean extract inhibits rat mammary carcinogenesis through BRCA1 and TNF-α expression: In silico and in vivo study." Open Veterinary Journal 14 (2024), 2678-2686. doi:10.5455/OVJ.2024.v14.i10.17 MLA (The Modern Language Association) Style Pratama, Dyah Ayu Oa, Annesia Fernanda, Ricadonna Raissa, Fajar Shodiq Permata, and Muhammad Luqman Nordin. "Black soybean extract inhibits rat mammary carcinogenesis through BRCA1 and TNF-α expression: In silico and in vivo study." Open Veterinary Journal 14.10 (2024), 2678-2686. Print. doi:10.5455/OVJ.2024.v14.i10.17 APA (American Psychological Association) Style Pratama, D. A. O., Fernanda, . A., Raissa, . R., Permata, . F. S. & Nordin, . M. L. (2024) Black soybean extract inhibits rat mammary carcinogenesis through BRCA1 and TNF-α expression: In silico and in vivo study. Open Veterinary Journal, 14 (10), 2678-2686. doi:10.5455/OVJ.2024.v14.i10.17 |