| Research Article | ||
Open Vet. J.. 2024; 14(10): 2572-2586 Open Veterinary Journal, (2024), Vol. 14(10): 2572–2586 Research Article Immune response and bacterial resistance of Oreochromis niloticus against bacterial fish pathogen with saffron dietHeba A. Tolba1, Ahmed M. Aldawek2, Refaat A. Eid3, Sherine Aladdin4 and Nahla H. El-Shaer5*1Central Laboratory for Aquaculture Research (CLAR), Agriculture Research Center (ARC), Giza, Egypt 2Department of Pathology and Clinical Pathology, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya 3Department of Pathology, College of Medicine, King Khalid University, Abha, Saudi Arabia 4Department of Genetics, Faculty of Agriculture, Zagazig University, Zagazig, Egypt 5Department of Zoology, Faculty of Science, Zagazig University, Zagazig, Egypt *Corresponding Author: Nahla H. El-Shaer. Zoology Department, Faculty of Science, Zagazig University, Zagazig, Egypt. Email: nahlaelshaer [at] yahoo.com; nhelshaer [at] zu.edu.eg Submitted: 21/05/2024 Accepted: 14/09/2024 Published: 31/10/2024 © 2024 Open Veterinary Journal
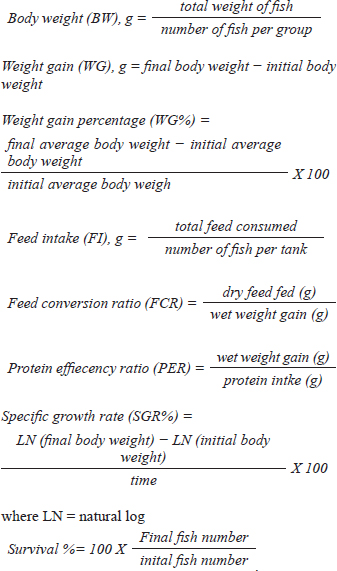
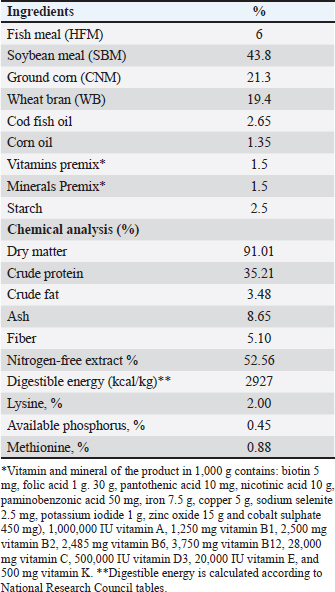
AbstractBackground: The global demand for fish and fish products has increased due to population growth and healthier food choices. However, bacterial infections caused by Aeromonas species pose a challenge. Antibiotics are crucial for disease control, but multidrug resistance is a global concern. Eco-friendly disease management methods, like saffron, have been identified as potential treatments. Aim: The study investigated the effects of dietary supplementation with Saffron on Nile tilapia's growth performance, immune response, and disease resistance. Methods: 180 fish were acclimatized for 2 weeks and randomly allocated into three groups. The first group served as a control, while the other two groups were fed a basal diet supplemented with Saffron at 1.5 g/kg (T1) and 0.5 g/kg (T2), respectively, for 12 weeks. Biochemical blood parameters. Histopathology and immunohistochemical studies were performed on the gills, liver, and spleen tissues. Results: Following the feeding trial with Saffron supplement, especially at higher levels enhanced weight gain, Growth performance, plasma total protein, and globulin showed higher levels in fish groups with dietary with Saffron at 1.5 g/kg (T1) and 0.5 g/kg (T2) than in fish fed the control diet Regulate the immune response in lysozyme activity and immunoglobulin M (IgM). Regeneration of gills, liver, and spleen tissues was noticed Furthermore, saffron-treated organs exhibited immunoreactivity to TNF-α was mostly seen in the liver and gills, although it was also somewhat in the kidney and spleen and CD68, the group were challenged with Aeromonas hydrophila, improved its defenses against A. hydrophila, immunity, and disease resistance than the control group. Conclusion: The results showed that saffron supplementation significantly increased the survival rate of fish challenged with A. hydrophila. It also enhanced the immune response of fish, as evidenced by increased levels of serum immunoglobulins and lysozyme activity. These findings suggest that saffron supplementation could be a promising strategy for the prevention and treatment of bacterial infections in aquaculture. Keywords: Histopathology, Immunohistochemistry, Nile tilapia, Saffron, Aeromonas hydrophila. IntroductionThe global demand for fish and fish products has been steadily increasing due to rapid population growth and a preference for healthier food options. Nile tilapia (Oreochromis niloticus) is a prominent species in freshwater aquaculture, with Egypt ranking third in its production worldwide (FAO, 2020). However, the aquaculture industry faces significant challenges, particularly in managing bacterial infections caused by Aeromonas species, which are ubiquitous gram-negative microbes commonly found in freshwater and estuary environments (Janda et al., 2010). These bacteria can be isolated from various sources, including water, soil, and food, and are normal inhabitants of fish gastrointestinal tracts (Sherif et al., 2023). The pathogenesis of Aeromonas is attributed to various virulence factors encoded by different genes (Dhooghe et al., 2011). Aeromonas hydrophila infection is particularly devastating, causing substantial financial losses in the global freshwater aquaculture sector (Hossain et al., 2014; Peterman et al., 2019). Antibiotics have been the cornerstone of bacterial disease control; however, the emergence of multidrug resistance has become a global concern (Cornaglia et al., 2011). Inappropriate use of antimicrobials in aquacultures, such as preventive treatments, has contributed to the development of antibiotic resistance in Aeromonas (Vivekanandhan et al., 2002; Sreedharan et al., 2012). Environmental factors and poor management practices in hatchery facilities can cause stress in aquatic animals, making them more vulnerable to infectious diseases (Winton, 2001). Disinfection used in hatcheries can disrupt the microbial balance, limiting the natural biological processes that control infections (Olafsen, 2001). Consequently, there is a growing interest in eco-friendly disease management methods to reduce the reliance on antibiotics and other therapeutic agents in aquaculture (Sherif et al., 2023). One such approach involves using saffron (Crocus sativus) a spice that has been used for centuries in traditional medicine. Additionally, the presence of α-crocin is primarily responsible for the herb's golden yellow-orange hue. The therapeutic properties of saffron are derived from its active metabolites, which include crocetin, crocin, safranal, and picrocrocin. (Attia et al., 2021). Saffron has a wide range of biological activities, including antibacterial, antifungal, and antioxidant properties. Wali et al. (2020) observed that the methanolic extract of saffron petals had significant antibacterial activity against Salmonella typhi, Bacillus cereus, Escherichia coli, Staphylococcus aureus, and Shigella dysenteriae. Saffron extracts have also been shown in another investigation to exhibit strong antibacterial activity against S. aureus and P. aeruginosa. The current study investigated the effect of saffron supplementation on the immune response and bacterial resistance of Nile tilapia challenged with A. hydrophila. Materials and MethodsExperimental designFish and acclimatization A total of 180 Nile tilapia (Oreochromis niloticus) with a mean body weight of 15 ± 5 g were randomly collected from earth ponds at Abbassa Fish Farm. The fish were acclimatized for 2 weeks in nine glass aquaria (60 × 50 × 70 cm) supplied with chlorine-free water and continuous aeration using air-pumping compressors. The water temperature was maintained at (22.5°C−28°C) and changed daily. Aqueous saffron extract preparation The dried stigmas of the saffron (Crocus sativus) flower, were purchased from a local market in Cairo, Egypt. 100 ml of distilled water was used to soak 1 g of saffron. After 2 hours, it was homogenized, stirred for 1 hour, and filtered. Fresh distilled water was used once more to remove the residue. Lyophilized, this aqueous extract was stored at 4°C until needed (Premkumar et al., 2003). Diets and feeding After the 2-week acclimation period, the experimental design consisted of three groups in triplicates (20 fish/tank): a control group and two experimental groups. The first group (T1) was a basal-fed diet supplemented with saffron (0.5 g)/kg commercial diets, the second group (T2) was fed a basal-fed diet supplemented with Saffron (1.5 g)/kg commercial diets, and the third group was fed a commercial non-medicated diet (control group) (C), containing 30% crude protein. The proximate chemical composition of the basal experimental diet was adjusted for Nile tilapia (O. niloticus) using the National Research Council's nutritional requirement according to (Sucu, 2020) presented in Table 1. Tilapia were fed with 3% of their total weight twice daily, for 12 weeks, fish from every group were weighed every 3 weeks, and the feed allowance was changed to reflect the new weight. The study was conducted at the Central Laboratory for Aquaculture Research, Alabassa, Abo-Hamad, Sharkia, Egypt, Agriculture Research Center, Ministry of Agriculture. Growth performance Weight gain, daily gain, and weight gain percentage were calculated using the following formulas according to (Zehra and Khan, 2011):
Table 1. Dietary content and proximate formulation of the Nile tilapia (Oreochromis nuloticus) basal diet.
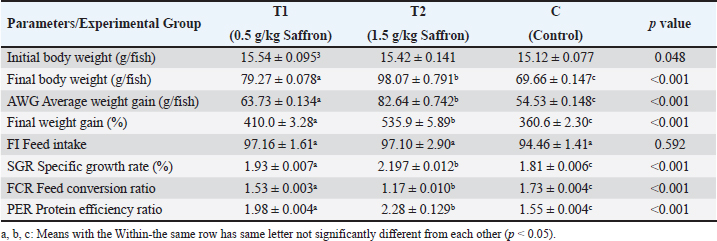
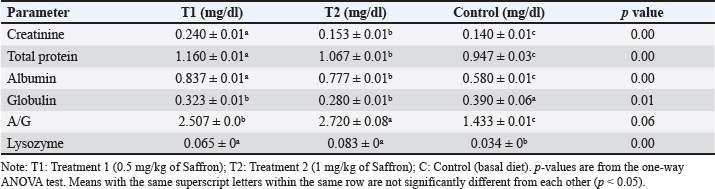
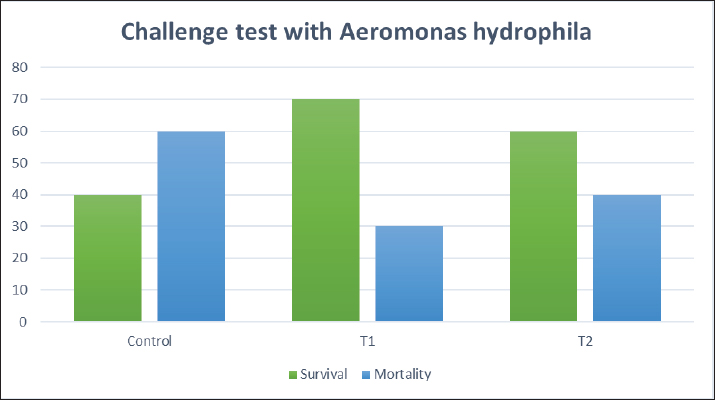
Biochemical blood parameters After 12 weeks, blood samples were taken from the caudal vessels of fish (five per replicate, 15 fish/treatment), with no anticoagulant. The blood was allowed to clot at room temperature for 2 hours before being centrifuged at 1,400 × g for ten minutes to extract serum, which was stored at −20°C until it was needed for further biochemical and immunological analysis. The total serum protein and albumin were determined according to (Chen et al., 2016), respectively. Globulin was calculated by subtracting albumin level from total protein, while serum creatinine was measured colorimetrically. Challenge test The isolation of Aeromonas species was done before the challenge, the strain was isolated from diseased Nile tilapia that suffered a normal hemorrhagic septicemia outbreak. A sample was collected from the surface and intestinal tract of fish under sterile conditions and squeezed into a sterile Eppendorf tube, which was then taken immediately for examination using the plate counting technique. The culture was selected and regularly cultured for 24 hours at 37°C on tryptic soy broth (Oxoid). After the bacterial colonies were purified, the morphology of the bacteria was determined by microscopic analysis of the cells and determination of the Gram type. Following the feeding trial, each treatment group was pooled and challenged by injection intraperitoneally with A. hydrophila at a dose of 0.1 ml of 24-hour broth containing 5 × 105 CFU/ml. A control group received 0.1 ml saline via intraperitoneal injection. Mortality and clinical signs Ten days post-injection mortality was recorded daily. The fish were also monitored for clinical signs of disease, such as loss of appetite, lethargy, skin lesions, and finally postmortem lesions. Then, survival %=100 × (final fish number/initial fish number) calculated according to Alandiyjany et al. (2022). Immunoglobulin Serum immunoglobulin M (IgM) levels were measured using an Enzyme-Linked Immunosorbent Assay (ELISA) kit and ELISA reader rt-2100c, MyBioSource.Com. Serum lysozyme activity Lysozyme activity was determined using the turbidometric assay (Biller et al., 2021). The units of lysozyme present in plasma or mucus (μg/ml) were obtained from a standard curve made with lyophilized hen-egg-white-lysozyme (Sigma). Histopathology After saffron treatment and 7 days post-challenge fishes were anesthetized by using a 0.1 ppm solution of ethyl 3-aminobenzoate methanesulfonate (Sigma-Aldrich Chemie GmbH, Eschenstrasse, product No. E10521, Germany), then fishes were necropsied, and the liver, kidneys, gills, and spleen were removed. The tissues were fixed in 10% formalin and processed for histopathology Specimens were then immediately processed to obtain 4 μm paraffin section cut from both sagittal and transverse planes. Sections were stained with Hematoxylin and Eosin stain for microscopic examination. Immunohistochemistry The immunohistochemistry analysis was carried out using standard procedures (Vannucchi, 2022). The 5 µm-thick paraffin sections were deparaffinized in Xylene and rehydrated by immersing the slides through alcohol grades step, then antigen retrieval done using a microwave and bring all slides to a boil in 10 mM sodium citrate buffer (pH 6.0), then tissue sections immersed in 3.0% hydrogen peroxide in methanol for 15 minutes, to block endogenous peroxidase activity, then the primary antibody (TNFα, NBP1-19532, dilution: 1/250) and (CD68, NB100-683, dilution: 1/1000) from Novus Biologicals (Littleton, CO, USA) incubated at room temperature for 1–2 hours. Continued the incubation overnight at 4°C in a humidified chamber. For detection, 100–400 ul Streptavidin-horseradish peroxidase reagent was added to each section and incubated for 30 minutes at room temperature, followed by 100–400 ul 3,3′-diaminobenzidine tetrahydrochloride substrate was used as chromogen, then counterstained sections in hematoxylin, dehydrated and mounted. The study used an Olympus digital camera and microscope to digitize image analysis slides of both TNFα and CD68 positive expression. The images were analyzed using Video Test Morphology 5.2 software on an Intel® Core I3®-based computer. The system measured the area percentage of Caspase-3 positive expression. Statistical analysis Data were statistically analyzed using the SPSS software version 23. A one-way ANOVA test was performed for comparison between different treatments, and a two-way ANOVA test was performed for comparison between different treatments, different times, and their interaction. Duncan's multiple ranges test was used for comparisons between means of groups. The means followed by the same letter were not significantly different from each other at the 5% probability level (p-value at 0.05). Ethical approval All experimental techniques were carried out according to the guidelines and suggestions approved by the Institutional Animal Care and Use Committee (IACUC), Faculty of Science, Zagazig University, Egypt, using the reference number (ZU-IACUC/1/F/407/2022). ResultsGrowth performance and feed efficiencyThe effects of saffron supplementation on the growth performance of Nile tilapia (Oreochromis niloticus) are presented in (Table 2). Fish supplemented with 1.5 g/kg saffron (Group T1) showed significantly (p < 0.05) higher average weight gain (AWG) compared to the control and 0.5 g/kg saffron treated group (82.64 g, 63.73 g, and 54.53 g, respectively). Moreover, the 1.5 g/kg saffron (Group T1) groups showed the greatest improvement (p < 0.05) in FCR, protein efficiency ratio (PER), and SGR than the control and 0.5 g/kg saffron treated group (Group T2). The control group showed the lowest growth performance parameters (AWG, SGR, FCR, and FER). Biochemical blood parametersThe effects of Saffron supplementation on biochemical parameters in Nile tilapia are shown in (Table 3). In terms of creatinine levels, the T1 and T2 groups showed significantly higher levels (0.240 ± 0.01 and 0.153 ± 0.01 mg/dl, respectively) compared to the control group (0.140 mg/dl). The total protein levels were significantly higher in the T1 and T2 groups (1.160 ± 0.01 and 1.067 ± 0.01 g/dl, respectively) compared to the control group (0.947 ± 0.03 g/dl). The albumin levels in the T1 and T2 groups were significantly higher (0.837 ± 0.01 and 0.777 ± 0.01 g/dl, respectively) compared to the control group (0.580 ± 0.01g/dl). The T1 and T2 groups also showed significant differences in terms of globulin levels, with values of 0.323 ± 0.01 and 0.280 ± 0.01 g/dl, respectively, compared to the control group (0.390 ± 0.06 g/dl). Additionally, the albumin/globulin (A/G) ratio was significantly higher in the T1 and T2 groups (2.507 ± 0.0 and 2.720 ± 0.08, respectively) compared to the control group (1.433 ± 0.01). The comparison between the control group and the Saffron-treated groups revealed that the T1 and T2 groups had significantly higher levels of creatinine, total protein, albumin, globulin, and lysozyme activity than the control group. However, there were no significant differences between the two Saffron-treated groups for most of the parameters except for creatinine, where the T2 group had significantly higher levels than the T1 group. Challenge test with A. hydrophilaSymptoms such as loss of scales and bleeding at the caudal peduncle area were observed in the clinically challenged fish of the control groups, the control group had a survival rate of 40% and a mortality rate of 60%. Mild hemorrhaging at the caudal peduncle in fish that otherwise appeared healthy. So, the group fed with Saffron at a dose of 1.5 g/kg (T1) exhibited significantly reduced symptom severity following A. hydrophila challenge compared to other groups had a survival rate of 70% and a mortality rate of 30%. The group fed with Saffron at a dose of 0.5 g/kg (T2) show also, decreased severity and had a survival rate of 60% and a mortality rate of 40% (Fig. 1). Table 2. Effect of two doses of saffron on growth performance of Nile tilapia (mean ± SE).
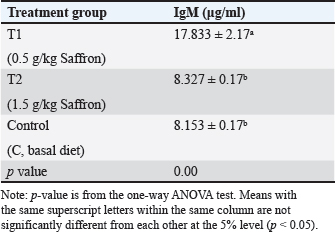
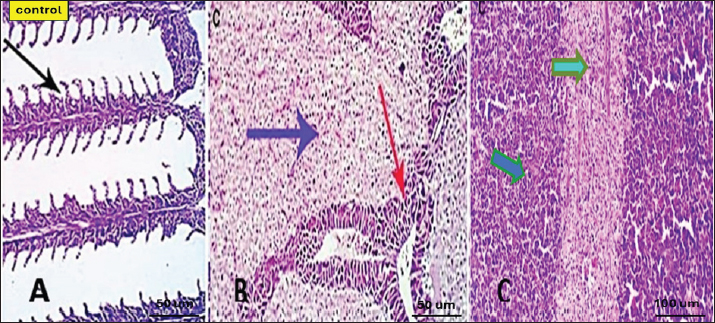
Serum lysozyme activityThe results in Table 3 showed that Saffron supplementation had a significant effect on serum lysozyme activity. The T1 and T2 groups showed significantly higher lysozyme activity levels (0.065 ± 0 and 0.083 ± 0, respectively) as compared to the control group (0.034 ± 0) (Table 3). Total IgMThe results indicate that the serum IgM levels in the experimental groups (T1 and T2) were significantly different from the control group (C). The mean serum IgM level in group T1 was (17.833 ± 2.17), which was significantly higher than the control group (8.153 ± 0.17). Similarly, the mean serum IgM level in group T2 was (8.327 ± 0.17), which was also significantly higher than the control group (8.153 ± 0.17). Additionally, the percentage of serum IgM in the experimental groups (T1 and T2) was significantly higher than in the control group (Table 4). Histopathological findingsControl group Microscopic examination of the gills, liver, and spleen of control fish revealed normal histological structures. Gill filaments showed normal primary and secondary lamellae with normal epithelial lining (Fig. 2A). Liver showed normal hepatic cords, hepatopancreas, and bile ducts (Fig. 2B). Spleen showed normal lymphoid follicles and melano-macrophage centers with no remarkable changes (Fig. 2C). Table 3. Effect of two doses of saffron on biochemical parameters and serum lysozyme activity in O. niloticus (mean ± SE).
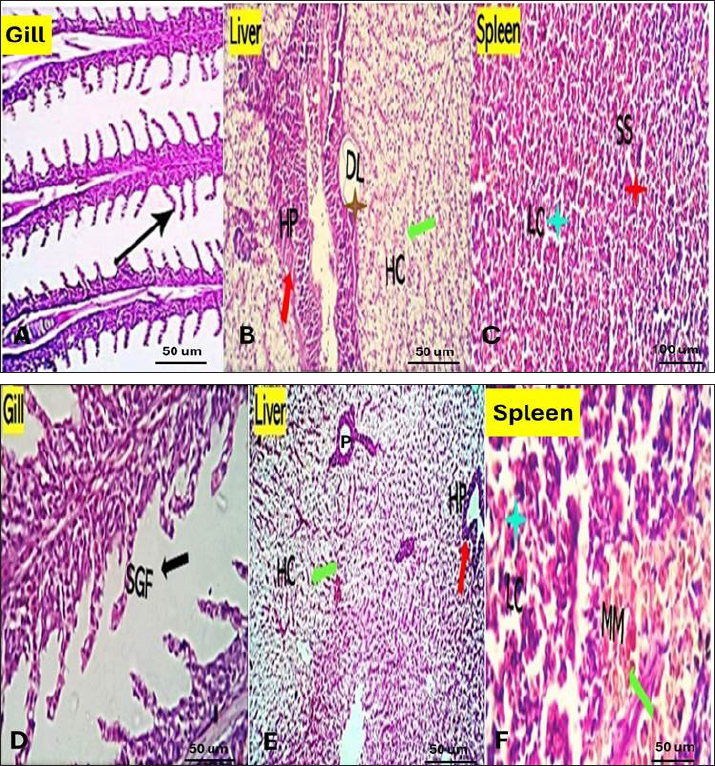
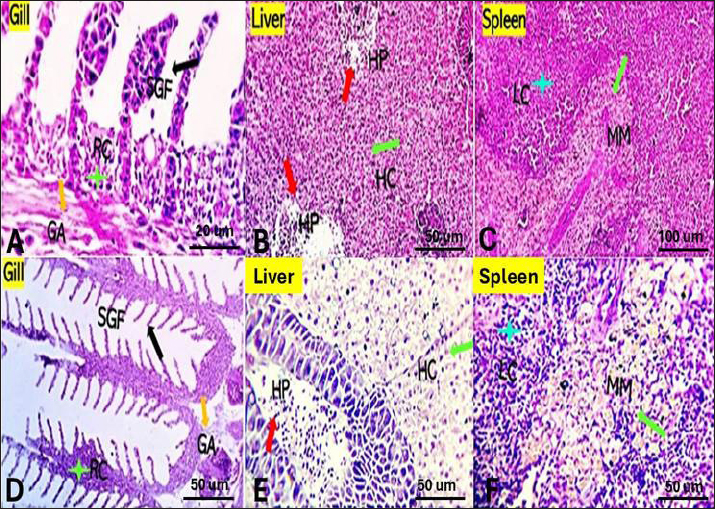
Fig. 1. The survival rate of the fish after being challenged with A. hydrophila. Saffron prophylactic groups (1.5 (T1) and 0.5 (T2) g/kg BW) The gills of fish of both treated groups did not show any aberration throughout the experiment, and the filaments and gill lamellae were well synchronized with inter-lamellar space in (T1) treatment group (Fig. 3A). However, some of the gill abnormalities appeared with (T2) saffron treatment group (Fig. 3D). The liver in (T1) had a more normal histological appearance. Hepatocytes virtually kept their polygonal structure with spherical nuclei and their regular orientation in cords (Fig. 3B), as well as in (T2) a more intact hepatopancreas contact with hepatocytes and the pancreatic acini, which are made up of pancreatic cells and afferent blood arteries, have been found to liver of (T2) group (Fig. 3E). The spleen of (T1) treated saffron group (Fig. 3C) had the typical look of red and white pulp. The (T2) spleen showed mildly dilated sinusoids and mildly activated melano-macrophage centers (Fig. 3F). Table 4. Effect of two doses of saffron on serum Immunoglobulin (IgM) after challenge with Aeromonas hydrophilia in O. niloticus (mean ± SE).
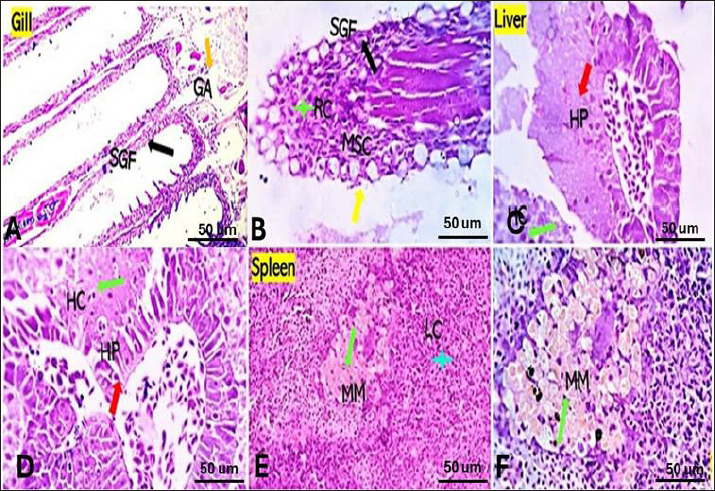
Bacterial challenged group Gills showed partial replacement and focal proliferation of secondary lamellae with round cell infiltration and focal necrosis. Mucus-secreting cells (MSC) were observed (Fig. 4A). Liver showed focal hepatocellular degeneration, necrosis, and vascular dilation. The pancreas showed degenerative, apoptotic, and necrotic changes (Fig. 4B). Spleen showed diffuse lymphoid depletion, vascular dilation, focal hemorrhages, and marked activation of melano-macrophage centers (Fig. 4C). Saffron-challenged groups (1.5 (T1) and 0.5 (T2) g/kg BW) Gills of (T1) treated group showed a low degree of focal denudation and adhesion of secondary lamellae with telangiectatic capillaries and round cell infiltration. The apical ends of secondary filaments were almost recovered (Fig. 5A), the erosion of cells in some areas decreased, and granular cell infiltration in gill arches (Fig. 5D). The spongy aspect of the liver was improved in both saffron-treated groups, especially (T1) treated group (Fig. 5B). There were no serious anomalies in (T2) saffron treatment group, liver exhibited considerable vacuolization, blood congestion, normal histology (Fig. 5E). The splenic tissue has relatively normal structure, with an increases melanomacrophages and lymphocyte infiltration with a higher dose of saffron (T1) group (Fig. 5C). Spleen of (T2) treatment group showed also the normal appearance of red and white pulp with moderately activated Milano-macrophage centers in (Fig. 5F).
Fig. 2. Photomicrograph of control gills, liver, and spleen showing normal micromorphological structures of the corresponding organs with preserved gill filament structures (black arrows) in A, normally arranged hepatic cords (blue arrow), normal hepatopancreas (red arrow) in B, and apparently normal splenic lymphoid elements (green arrow) and mildly activated Milano-macrophage centers (Light blue arrow) in C.
Fig. 3. Gills, Liver, and Spleen of Saffron (Crocus sativus) prophylaxis dose ( 1.5, 0.5 /Kg BW), respectively, in gills showing normal secondary filament structures in A partial adhesion of the secondary filaments are seen in (SGF, black arrow) in D. In liver the pancreatic cells (P) and afferent blood arteries, have been found to be attached to the liver. Mildly dilated lymphatics (DL, brown star) at the vicinity of the hepato-portal (HP) area and the hepatic cells (HC) in B and E. Spleen showing ill-distinctly distributed lymphoid elements (LC, light blue stars) around splenic sinusoids (SS, red star). The melano-macrophage centers (MMC,green arrow) are mildly activated, in C and F.
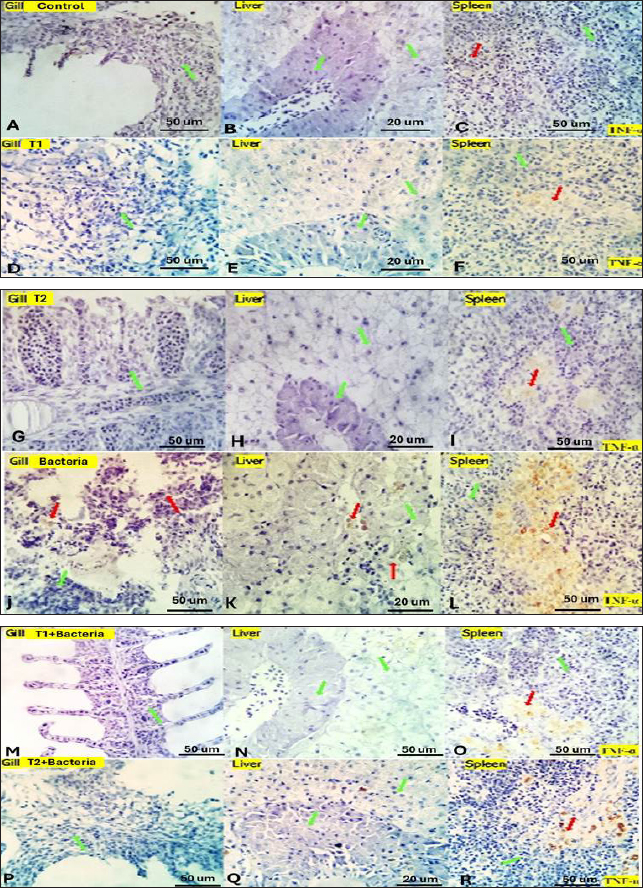
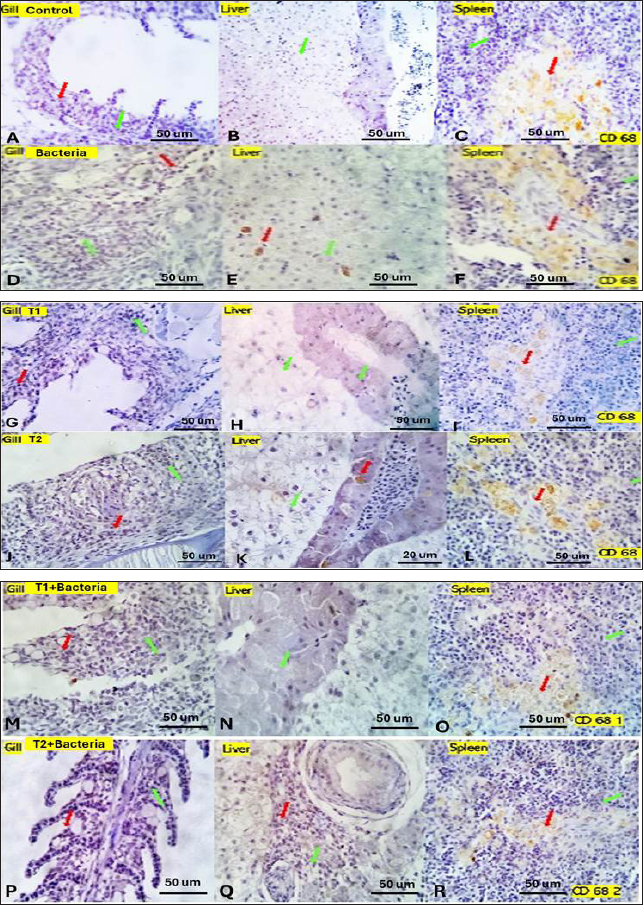
Fig. 4. Gills, Liver, and Spleen of bacterial-challenged fish showing focal epithelial proliferative changes (black arrow) with partial replacement of the secondary filaments. Round cell infiltration is also seen (RC, green star). Some of the gill filaments show focal and or total epithelial necrosis with reactive MSC in A and B. Hepatic tissue showing focal hepatocellular degenerative and early necrotic changes (HC green arrow). Focal vascular dilation, hepato-pancreatic degenerative, apoptotic, and necrotic changes (HP red arrow) are seen in C and D. Splenic shows diffuse lymphoid depletion (LC light blue star), vascular dilatation, focal hemorrhages, and marked activation of the melano-macrophage centers (Green arrow) in E and F. ImmunohistochemistryTumor necrosis factor-alpha (TNF-α) expression The control group showed negative expression in gills and liver and mild expression in Splenic melano-macrophages (6.4%) (Fig. 6A, B, C). The bacterial-challenged group showed expression in a few cells of the gills (4.7%) and liver (1.4%) and moderate expression in splenic melano-macrophages (20%) (Fig. 6J, K, L). Saffron prophylactic groups (T1 and T2) showed expression in a few splenic melano-macrophages (9.8 and 9.4%), respectively, (Fig. 6D, E, F) and (Fig. 6G, H, I), saffron-challenged groups after T1 and T2 treatment, respectively, showed very few positive cells in spleen (1.8 and 1.22%) and negative expression in gills and liver (Fig. 6M, N, O) and (Fig. 6P, Q, R). Macrophages (CD68) expression The control group showed few macrophages (CD68 positive) cells in the gills (3%) and liver (2.5%) and mild to moderate expression in splenic melano-macrophages (16.4%) (Fig. 7A, B, C). The bacterial-challenged group showed expression in a few cells of gills (4.8%) and liver (3%) and moderate expression in splenic melano-macrophages (27.6%) (Fig. 7E, F, G). Saffron prophylactic groups (T1 and T2) showed expression in some splenic melano-macrophages (6.3 and 6.8%) and few positive cells in gills (1.67 and 0.88%) and liver (4.2% and 1.12%), respectively, (Fig. 7H, I, J) and (Fig. 7K, L, M). Saffron-challenged groups after T1 and T2 treatment, respectively, showed expression in few splenic melano-macrophages (18.1% and 8.9%) and very few positive cells in gills (1.76% and 0.23%) and liver (0.25% and 0.13%) (Fig. 7N, O, P) and (Fig. 7Q, R, S). DiscussionSaffron (Crocus sativus), a Middle Eastern herb, is known for its potent antioxidant properties due to constituents such as safranal, crocin, and crocetin (Broadhead et al., 2016). It possesses various pharmacological properties, including antitumor (Moradzadeh et al., 2019), antioxidant (Armellini et al., 2018), anti-inflammatory (Ashktorab et al., 2019), antispasmodic, expectorant, antifungal, antibacterial, antiseptic (Schmidt et al., 2007; Mollazadeh et al., 2015), hypolipidemic (Ghaffari et al., 2019), antidepressant (Lopresti et al., 2017), and antihypertensive effects (Naim et al., 2023). The antimicrobial activities are primarily attributed to safranal and crocin compounds (Carmona et al., 2007).
Fig. 5. Gills, Liver, and Spleen of post bacterial challenge and saffron (Crocus sativus) treatment (1.5, 0.5 /Kg BW), respectively, showing focal denudation of the secondary gill filament lining epithelium (SGF, black arrow), thickening and adhesion of the secondary filaments due to round cells infiltration, mostly macrophages and lymphocytes (RC, green star) and eosinophils granular cells infiltration in the gill arch (GA, orange arrow) are seen in both treatment A and D, normal hepato-portal pancreatic structures (HP, red arrow) and normal hepatocellular structural morphology (HC, green arrow) are seen in the Liver of B and E Moderately activated melano-macrophage centers (MMC, green arrow), and apparently, normal lymphoid elements (LC) are seen in Spleen of C and F. While Saffron's antioxidant activity in biological systems is well-documented (Parray et al., 2015; Okmen et al., 2016), its effects on food products have received limited attention (Najafi et al., 2022). One study reported delayed oxidation in shrimp and demonstrated antibacterial activity (Aboutorab et al., 2021). Research on saffron supplementation in aquatic animals is little, with no studies conducted on Nile tilapia. The present study is the first to investigate the effects of Saffron on Nile tilapia performance. Herbals' active components can induce digestive enzyme secretion, aiding carbohydrate, and protein biodegradation (Citarasu, 2010). The results of the study show that saffron supplementation significantly improved the growth performance of Nile tilapia. Fish supplemented with 0.5 g/kg saffron had a significantly higher AWG than the control group, and they also had a better feed conversion ratio (FCR) and feed efficiency ratio. The improved growth performance and survival rate of tilapia supplemented with saffron may be linked to enhanced nutrient availability, feed utilization, and digestive enzyme activity. Saffron may enhance growth through its antioxidant and anti-inflammatory effects, protecting fish from stress and disease. Additionally, saffron could improve fish metabolism, leading to faster growth. Saffron extract has been shown to increase gastric acid and pepsin secretions in rats, possibly through nitric oxide increment (Nabavizadeh et al., 2009).
Fig. 6. Gills, liver and spleen of different experimental groups, immune-stained by TNF-α, showing the positively reacted inflammatory cells (red arrows) and the negatively stained cells (green arrows).
Fig. 7. Gills, Liver, and Spleen of different experimental groups, immune-stained by CD 68, showing the positively reacted inflammatory cells (red arrows) and the negatively stained cells (green arrows). Similar to our result, Hassan et al. (2018) reported among fish that herbal additives, such as turmeric, rosemary, and thyme, have been shown to improve FCR and protein efficiency ratio (PER), potentially due to their ability to enhance fat metabolism and utilization. These plants also possess high antioxidant activity, which may contribute to the activation of immune functions in fish (Ji et al., 2007). Furthermore, herbal extracts have been reported to reduce mortality against pathogenic challenges in fish (Jian et al., 2003). Unlike our findings, a study found that saffron and crocin significantly reduced body weight, food intake, and blood leptin levels in adult male Wistar rats compared to the control group and baseline (Kianmehr et al., 2022). Concerning the serum biochemical parameters, saffron supplementation significantly increased serum total protein, albumin, globulin, and lysozyme activity compared to the control group, indicating improved protein biosynthesis, metabolism, liver function, and antibody production (Sivaram et al., 2004). The elevated serum protein levels, particularly globulin and lysozyme activity, suggest enhanced immune function in fish fed with plant extracts (Misra et al., 2006; Marin et al., 2015). Our results indicate that saffron supplementation, especially at 0.5 g/kg, positively impacted serum protein levels, particularly globulin, as well as serum lysozyme activity, which may contribute to enhanced immune function in Nile tilapia. The lower albumin/globulin ratio in T2 suggests greater humoral immune system activity in this group. Similarly, elevated serum total protein levels can be linked to the innate immune response in fish that are fed on diets typically enhanced with immunostimulants (Wiegertjes et al., 1996; Rudneva et al., 2011). Immunostimulants can activate immune systems by enhancing the number of phagocytes, activating phagocytes, or increasing the synthesis of involved molecules. In a study by Hassan et al. (2018), fish fed with turmeric, rosemary, and thyme exhibited elevated phagocytic activity, suggesting a stronger innate response (Nayak et al., 2004). Using natural plant products as immunostimulants in fish farming is considered more advantageous than antibacterial drugs, as they avoid potential adverse side effects for fish, the environment, and consumers (Hassan et al., 2018). Lysozyme, an enzyme with phagocytic and antimicrobial properties, plays a crucial role in the innate immune response of fish (Alexander et al., 1992; Magnadottir, 2010). The increased serum lysozyme activity observed in the current study indicates the beneficial use of saffron supplementation in the Nile tilapia diet, promoting innate immunity against bacterial infections and pathogens. Other studies have also shown that chemical extracts of plant origin, containing bioactive molecules such as alkaloids, flavonoids, pigments, phenolics, terpenoids, and steroids, can enhance immunity and various biological activities in cultivated fish (Citarasu, 2010; Chakraborty et al., 2011, 2014). The challenge test with A. hydrophila demonstrated that the saffron-treated groups exhibited a higher survival rate of 70% and a mortality rate of 30% compared to the control group, indicating enhanced resistance to bacterial infections. This supports previous reports indicating increased resistance of fish against bacterial challenges when fed immunostimulants (Sahu et al., 2008; Harikrishnan et al., 2011). In terms of immunological parameters, saffron treatment resulted in significantly higher serum total IgM levels of 17.833 ± 2.17 compared to the control group. This increase in IgM levels highlights the stimulatory effects of saffron on antibody production. However, the percentage of IgM was significantly lower 8.327 ± 0.17 in the (T2) treated group, which could be attributed to the elevated levels of other serum proteins. Previous studies have also reported increased total serum IgM levels in fish supplemented with immunostimulants (Selvaraj et al., 2005; Sahu et al., 2008). Herbal extracts have been shown to enhance nonspecific immunity in fish by increasing phagocyte activity and serum immunoglobulin levels (Jian et al., 2003). These extracts have also demonstrated improved survival against pathogenic infections (Sivaram et al., 2004). The histopathological examination revealed normal histological structures of the gills, liver, and spleen in the control group. However, dietary supplementation of saffron at two different concentrations (0.5 and 1.5 g/kg feed) caused mild histological changes in these organs, especially at the higher dose (1.5 g/kg). In the gills, saffron treatment resulted in congested and telangiectatic capillaries with focal denudation and adhesion of secondary lamellae as well as round cell infiltration. These changes could be attributed to local inflammation and tissue damage in response to saffron intake, especially at higher concentrations. Similar gill lesions were reported in rainbow trout fed high doses of herbal extracts (Schwaiger et al., 2004; Tafi et al., 2020). However, the gill changes were milder at the lower saffron dose (0.5 g/kg), indicating limited toxicity effects at this concentration. In the liver, saffron supplementation caused mildly dilated lymphatics which may be linked to mild inflammatory reactions. The hepatopancreas showed complete necrosis in some fish fed 0.5 g/kg saffron. However, normal liver histology was also observed in some fish at this dose, suggesting limited toxicity, suggesting that treatment markedly suppressed the hepatotoxic lesions (Bukhari et al., 2018). These results indicate the importance of determining safe dosage levels when using natural extracts as feed supplements in aquaculture. In the spleen, saffron treatment resulted in mildly activated melano-macrophage centers and dilated sinusoids, which could be linked to the enhancement of the nonspecific immune response. Melano-macrophages play an important role in the immune defence mechanisms of fish. Their activation suggests stimulation of the splenic immune function by saffron supplementation. These immunostimulatory effects are in agreement with the results of serum lysozyme activity and total IgM that were previously discussed. The immunohistochemical examination revealed lowered expression of TNF-α and CD68 in the gills, liver, and spleen of saffron-treated groups compared to the bacteria-challenged group. TNF-α is a pro-inflammatory cytokine that plays a key role in initiating inflammatory and immune responses. Its decreased expression in saffron-treated fish suggests the potential anti-inflammatory effects of saffron. CD68 is a macrophage marker, so its lowered expression indicates reduced macrophage activity and tissue damage in the saffron groups compared to the infected fish. TNF-α is produced during the inflammatory response, leading to increased vascular penetration and activation of white blood cells at the site of infection or injury. This makes TNF the primary initiator of inflammation and blood vessel formation (Wei et al., 2010). Dietary saffron supplementation at a concentration of 0.5 g/kg showed limited toxicity effects and did not cause remarkable histological changes in the gills, liver, and spleen of Nile tilapia compared to the higher dose (1.5 g/kg). However, saffron treatment activated the nonspecific immune response as evidenced by mildly activated melano-macrophage centres in the spleen. Furthermore, saffron exhibited potential anti-inflammatory effects by lowering the expression of TNF-α and macrophage activity compared to the bacteria-challenged group. These results highlight the importance of determining the optimal dosage levels to achieve the immunostimulatory effects of natural supplements without causing major toxic impacts. ConclusionThe results of this study suggest that dietary saffron supplementation at a concentration of 0.5 g/kg can improve the growth performance, immune function, and anti-inflammatory properties of Nile tilapia. However, further studies are needed to investigate the optimal saffron supplementation dose and Saffron's long-term effects on Nile tilapia. AcknowledgmentsThe authors are grateful to the Faculty of Science, Zagazig University. Conflict of interestThe authors declare that there is no conflict of interest. FundingNone. Author ContributionsConceptualization, N.H.E., H.A., A.M.A, and R.A.E methodology, N.H.E., H.A., A.M.A., R.A.E, and SH.A.; validation, N.H.E., H.A., A.M.A., R.A.E.; investigation, N.H.E., H.A., R.A.E, and SH.A; resources, N.H.E., H.A., A.M.A., and R.A.E; N.H.E., H.A., A.M.A., R.A.E, and SH.A; data curation, N.H.E., H.A., A.M.A., R.A.E, and SH.A; writing—original draft preparation, N.H.E., H.A., A.M.A., R.A.E, and SH.A.; writing—review and editing, N.H.E., H.A., A.M.A., and R.A.E.; supervision, N.H.E., and A.M.A; All authors have read and agreed to the published version of the manuscript. Data availabilityAll data are provided in the manuscript. ReferencesAboutorab, M., Ahari, H., Allahyaribeik, S., Yousefi, S. and Motalebi, A. 2021. Nano-emulsion of saffron essential oil by spontaneous emulsification and ultrasonic homogenization extend the shelf life of shrimp (Crocus sativus L.). J. Food Process Preserv. 45, e15224. Alandiyjany, M.N., Kishawy, A.T., Abdelfattah-Hassan, A., Eldoumani, H., Elazab, S.T., El-Mandrawy, S.A. and Ibrahim, D. 2022. Nano-silica and magnetized-silica mitigated lead toxicity: their efficacy on bioaccumulation risk, performance, and apoptotic targeted genes in Nile tilapia (Oreochromis niloticus). Aquat. Toxicol. 242, 106054. Alexander, J.B. and Ingram, G.A. 1992. Noncellular nonspecific defence mechanisms of fish. Ann. Rev. Fish Dis. 2, 249–279. Armellini, R., Peinado, I., Pittia, P., Scampicchio, M., Heredia, A. and Andres, A. 2018. Effect of saffron (Crocus sativus L.) enrichment on antioxidant and sensorial properties of wheat flour pasta. Food Chem. 254, 55–63. Ashktorab, H., Soleimani, A., Singh, G., Amin, A., Tabtabaei, S., Latella, G., Stein, U., Akhondzadeh, S., Solanki, N., Gondré-Lewis, M.C., Habtezion, A. and Brim, H. 2019. Saffron: the golden spice with therapeutic properties on digestive diseases. Nutrients 11(5), 943. Attia, A.A., Ramdan, H.S., Al-Eisa, R.A., Adle Fadle, B.O. and El-Shenawy, N.S. 2021. Effect of saffron extract on the hepatotoxicity induced by copper nanoparticles in male mice. Molecules 26(10), 3045. Biller, J.D., Polycarpo, G.D.V. and Moromizato, B.S. 2021. Lysozyme activity as an indicator of innate immunity of tilapia (Oreochromis niloticus) when challenged with LPS and Streptococcus agalactiae. R. Bras. Zootec. 50,e20210053. Broadhead, G.K., Chang, A., Grigg, J. and Mccluskey, P. 2016. Efficacy and safety of saffron supplementation: current clinical findings. Crit. Rev. Food Sci. Nutr. 56, 2767–2776. Bukhari, S.I., Manzoor, M. and Dhar, M. 2018. A comprehensive review of the pharmacological potential of Crocus sativus and its bioactive apocarotenoids. Biomed. Pharmacother. 98, 733–745. Carmona, M., Zalacain, A., Salinas, M.R. and Alonso, G.L. 2007. A new approach to saffron aroma. Crit. Rev. Food Sci. Nutr. 47, 145–159. Chakraborty, S.B. and Hancz, C. 2011. Application of phytochemicals as immunostimulant, antipathogenic and antistress agents in finfish culture. Rev. Aquac. 3, 103–119. Chakraborty, S.B., Horn, P. and Hancz, C. 2014. Application of phytochemicals as growth-promoters and endocrine modulators in fish culture. Rev. Aquac. 6, 1–19. Chen, X.-M., Guo, G.-L. and Sun, L. 2016. Effects of Ala-Gln feeding strategies on growth, metabolism, and crowding stress resistance of juvenile Cyprinus carpio var. Jian. Fish Shellfish Immunol. 51, 365–372. Citarasu, T. 2010. Herbal biomedicines: a new opportunity for aquaculture industry. Aquacult. Int. 18, 403–414. Cornaglia, G., Giamarellou, H. and Rossolini, G.M. 2011. Metallo-β-lactamases: a last frontier for β-lactams? Lancet Infect. Dis. 11, 381–393. Dhooghe, E., Van Laere, K., Eeckhaut, T., Leus, L. and Van Huylenbroeck, 2011. Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tissue Organ. Cult. 104, 359–373. FAO. 2020. The state of world fisheries and aquaculture sustainability in action. FAO, Rome, Italy, pp: 1–244. Ghaffari, S. and Roshanravan, N. 2019. Saffron: an updated review on biological properties with special focus on cardiovascular effects. Biomed. Pharmacother. 109, 21–27. Harikrishnan, R., Balasundaram, C. and Heo, M.-S. 2011. Impact of plant products on innate and adaptive immune system of cultured finfish and shellfish. Aquacult. 317, 1–15. Hassan, A.M., Yacout, M.H., Khalel, M.S., Hafsa, S.H.A., Ibrahim, M.R., Mocuta, D.N., Turek Rahoveanu, A. and Dediu, L. 2018. Effects of some herbal plant supplements on growth performance and the immune response in Nile tilapia (Oreochromis niloticus). Sciendo, 1, 134–141. Hossain, M.J., Sun, D., Mcgarey, D.J., Wrenn, S., Alexander, L.M., Martino, M.E., Xing, Y., Terhune, J.S. and Liles, M.R. 2014. An Asian origin of virulent Aeromonas hydrophila responsible for disease epidemics in United States-farmed catfish. MBio. 5, e00848–14. Janda, J.M. and Abbott, S.L. 2010. The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 23, 35–73. Ji, S.-C., Takaoka, O., Jeong, G.-S., Lee, S.-W., Ishimaru, K., Seoka, M. and Takii, K. 2007. Dietary medicinal herbs improve growth and some non-specific immunity of red sea bream Pagrus major. Fish Sci. 73, 63–69. Jian, J. and Wu, Z. 2003. Effects of traditional Chinese medicine on nonspecific immunity and disease resistance of large yellow croaker, Pseudosciaena crocea (Richardson). Aquacult. 218, 1–9. Kianmehr, M., Mahdizadeh, F. and Khazdair, M.R. 2022. The effects of Crocus sativus L. (Saffron) and its ingredients on dietary intakes in cardiovascular disease in Iranian population: a systematic review and meta-analysis. Front. Nutr. 9, 890532. Lopresti, A.L. and Drummond, P.D. 2017. Efficacy of curcumin, and a saffron/curcumin combination for the treatment of major depression: a randomised, double-blind, placebo-controlled study. J. Affect. Disord. 207, 188–196. Magnadottir, B. 2010. Immunological control of fish diseases. Mar. Biotechnol. 12, 361–379. Marin, M., Nicolae, C., Drăgotoiu, D., Urdeş, L., Răducuță, I. and Diniță, G. 2015. Researches regarding the haematological profile of juvenile Cyprinus carpio varieties. Sci. Papers Ser. D. Anim. Sci. 58, 209–212. Misra, C.K., Das, B.K., Mukherjee, S.C. and Pattnaik, P. 2006. Effect of long term administration of dietary β-glucan on immunity, growth and survival of Labeo rohita fingerlings. Aquacult. 255, 82–94. Mollazadeh, H., Emami, S.A. and Hosseinzadeh, H. 2015. Razi's Al-Hawi and saffron (Crocus sativus): a review. Iran. J. Basic Med. Sci. 18, 1153–1166. Moradzadeh, M., Kalani, M.R. and Avan, A. 2019. The antileukemic effects of saffron (Crocus sativus L.) and its related molecular targets: a mini review. J. Cell Biochem. 120, 4732–4738. Nabavizadeh, F., Salimi, E., Sadroleslami, Z., Karimian, S.M. and Vahedian, J. 2009. Saffron (Crocus sativus) increases gastric acid and pepsin secretions in rats: role of nitric oxide (NO). Afr. J. Pharm. Pharmacol. 3, 181–184. Naim, N., Bouymajane, A., Oulad El Majdoub, Y., Ezrari, S., Lahlali, R., Tahiri, A., Ennahli, S., Laganà Vinci, R., Cacciola, F., Mondello, L. and Madani, I. 2023. Flavonoid composition and antibacterial properties of Crocus sativus L. petal extracts. Molecules 28, 186. Najafi, Z., Cetinkaya, T., Bildik, F., Altay, F. and Yeşilçubuk, N.Ş. 2022. Nanoencapsulation of saffron (Crocus sativus L.) extract in zein nanofibers and their application for the preservation of sea bass fillets. LWT. 163, 113588. Nayak, A., Das, B., Kohli, M. and Mukherjee, S. 2004. The immunosuppressive effect of α-permethrin on Indian major carp, rohu (Labeo rohita Ham.). Fish Shellfish Immunol. 16, 41–50. Okmen, G., Kardas, S., Bayrak, D., Arslan, A. and Cakar, H. 2016. The antibacterial activities of Crocus sativus against mastitis pathogens and its antioxidant activities. World J. Pharm. Res. 5, 146–156. Olafsen, J.A. 2001. Interactions between fish larvae and bacteria in marine aquaculture. Aquacult. 200, 223–247. Parray, J.A., Kamili, A.N., Hamid, R., Reshi, Z.A. and Qadri, R.A. 2015. Antibacterial and antioxidant activity of methanol extracts of Crocus sativus L. cv. Kashmirianus. Front. Life Sci. 8, 40–46. Peterman, M.A. and Posadas, B.C. 2019. Direct economic impact of fish diseases on the East Mississippi catfish industry. N. Am. J. Aquac. 81, 222–229. Premkumar, K., Abraham, S.K., Santhiya, S.T. and Ramesh, A. 2003. Protective effects of saffron (Crocus sativus Linn.) on genotoxins-induced oxidative stress in Swiss albino mice. Phytother. Res. 17, 614–617. Rudneva, I.I. and Kovyrshina, T.B. 2011. Comparative study of electrophoretic characteristics of serum albumin of round goby Neogobius melanostomus from Black Sea and Azov Sea. Int. J. Sci. Nat. 1, 131–136. Sahu, S., Das, B.K., Mishra, B.K., Pradhan, J., Samal, S.K. and Sarangi, N. 2008. Effect of dietary Curcuma longa on enzymatic and immunological profiles of rohu, Labeo rohita (Ham.), infected with Aeromonas hydrophila. Aquac. Res. 39. 1720–1730. Schmidt, M., Betti, G. and Hensel, A. 2007. Saffron in phytotherapy: pharmacology and clinical uses. Wien. Med. Wochenschr. 157, 315–319. Schwaiger, J., Ferling, H., Mallow, U., Wintermayr, H. and Negele, R. 2004. Toxic effects of the non-steroidal anti-inflammatory drug diclofenac: part I: histopathological alterations and bioaccumulation in rainbow trout. Aquat. Toxicol. 68, 141–150. Selvaraj, V., Sampath, K. and Sekar, V. 2005. Administration of yeast glucan enhances survival and some non-specific and specific immune parameters in carp (Cyprinus carpio) infected with Aeromonas hydrophila. Fish Shellfish Immunol. 19, 293–306. Sherif, A.H. and Kassab, A.S. 2023. Multidrug-resistant Aeromonas bacteria prevalence in Nile tilapia broodstock. BMC Microbiol. 23, 80. Sivaram, V., Babu, M., Immanuel, G., Murugadass, S., Citarasu, T. and Marian, M.P. 2004. Growth and immune response of juvenile greasy groupers (Epinephelus tauvina) fed with herbal antibacterial active principle supplemented diets against Vibrio harveyi infections. Aquacult. 237, 9–20. Sucu, E. 2020. Effects of microalgae species on in vitro rumen fermentation pattern and methane production. Ann. Anim. Sci. 20(1), 207–218. Sreedharan, K., Philip, R. and Singh, I.S. 2012. Virulence potential and antibiotic susceptibility pattern of motile aeromonads associated with freshwater ornamental fish culture systems: a possible threat to public health. Braz. J. Microbiol. 43, 754–765. Tafi, A., Meshkini, S., Tukmechi, A., Alishahi, M. and Noori, F. 2020. Therapeutic and histopathological effect of Aloe vera and Salvia officinalis hydroethanolic extracts against Streptococcus iniae in Rainbow Trout. Arch. Razi. Institute 75, 275. Vannucchi, M.G. 2022. Telocytes and macrophages in the gut: from morphology to function, do the two cell types interact with each other? Which helps which? Int. J. Mol. Sci. 23(15), 8435. Vivekanandhan, G., Savithamani, K., Hatha, A.A. and Lakshmanaperumalsamy, P. 2002. Antibiotic resistance of Aeromonas hydrophila isolated from marketed fish and prawn of South India. Int. J. Food Microbiol. 76, 165–168. Wali, A.F., Alchamat, H.A.A., Hariri, H.K., Hariri, B.K., Menezes, G.A., Zehra, U. and Ahmad, P. 2020. Antioxidant, antimicrobial, antidiabetic and cytotoxic activity of Crocus sativus L. petals. Appl. Sci. 10(4), 1519. Wei, L., Jia, L., Jiayuan, B., Changqin, G., Wanpo, Z., Guofu, C. and Xueying, H. 2010. Recent progress of tumor necrosis factor-α. Dong. Wu. Yi. Xue. Fa. Zhan. 31, 108–111. Wiegertjes, G.F., Stet, R.J., Parmentier, H.K. and Van Muiswinkel, W.B. 1996. Immunogenetics of disease resistance in fish: a comparative approach. Dev. Comp. Immunol. 20, 365–381. Winton, J.R. 2001. Fish health management fish hatchery management, 2nd ed. American Fisheries Society, Bethesda, Maryland, USA. Zehra, S. and Khan, M. A. 2011. Dietary protein requirement for fingerling Channa punctatus (Bloch), based on growth, feed conversion, protein retention and biochemical composition. Aquac. Int. 20(2), 383–395. | ||
| How to Cite this Article |
| Pubmed Style Tolba HA, Aldawek AM, Eid RA, Aladdin S, El-shaer NH. Immune response and bacterial resistance of Oreochromis niloticus against bacterial fish pathogen with saffron diet. Open Vet. J.. 2024; 14(10): 2572-2586. doi:10.5455/OVJ.2024.v14.i10.7 Web Style Tolba HA, Aldawek AM, Eid RA, Aladdin S, El-shaer NH. Immune response and bacterial resistance of Oreochromis niloticus against bacterial fish pathogen with saffron diet. https://www.openveterinaryjournal.com/?mno=200658 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i10.7 AMA (American Medical Association) Style Tolba HA, Aldawek AM, Eid RA, Aladdin S, El-shaer NH. Immune response and bacterial resistance of Oreochromis niloticus against bacterial fish pathogen with saffron diet. Open Vet. J.. 2024; 14(10): 2572-2586. doi:10.5455/OVJ.2024.v14.i10.7 Vancouver/ICMJE Style Tolba HA, Aldawek AM, Eid RA, Aladdin S, El-shaer NH. Immune response and bacterial resistance of Oreochromis niloticus against bacterial fish pathogen with saffron diet. Open Vet. J.. (2024), [cited January 25, 2026]; 14(10): 2572-2586. doi:10.5455/OVJ.2024.v14.i10.7 Harvard Style Tolba, H. A., Aldawek, . A. M., Eid, . R. A., Aladdin, . S. & El-shaer, . N. H. (2024) Immune response and bacterial resistance of Oreochromis niloticus against bacterial fish pathogen with saffron diet. Open Vet. J., 14 (10), 2572-2586. doi:10.5455/OVJ.2024.v14.i10.7 Turabian Style Tolba, Heba A., Ahmed M. Aldawek, Refaat A. Eid, Sherine Aladdin, and Nahla H. El-shaer. 2024. Immune response and bacterial resistance of Oreochromis niloticus against bacterial fish pathogen with saffron diet. Open Veterinary Journal, 14 (10), 2572-2586. doi:10.5455/OVJ.2024.v14.i10.7 Chicago Style Tolba, Heba A., Ahmed M. Aldawek, Refaat A. Eid, Sherine Aladdin, and Nahla H. El-shaer. "Immune response and bacterial resistance of Oreochromis niloticus against bacterial fish pathogen with saffron diet." Open Veterinary Journal 14 (2024), 2572-2586. doi:10.5455/OVJ.2024.v14.i10.7 MLA (The Modern Language Association) Style Tolba, Heba A., Ahmed M. Aldawek, Refaat A. Eid, Sherine Aladdin, and Nahla H. El-shaer. "Immune response and bacterial resistance of Oreochromis niloticus against bacterial fish pathogen with saffron diet." Open Veterinary Journal 14.10 (2024), 2572-2586. Print. doi:10.5455/OVJ.2024.v14.i10.7 APA (American Psychological Association) Style Tolba, H. A., Aldawek, . A. M., Eid, . R. A., Aladdin, . S. & El-shaer, . N. H. (2024) Immune response and bacterial resistance of Oreochromis niloticus against bacterial fish pathogen with saffron diet. Open Veterinary Journal, 14 (10), 2572-2586. doi:10.5455/OVJ.2024.v14.i10.7 |