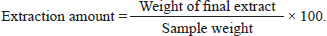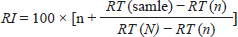| Research Article | ||
Open Vet. J.. 2024; 14(10): 2564-2571 Open Veterinary Journal, (2024), Vol. 14(10): 2564–2571 Research Article The application of pheromones extracted from Shami goats' male hair and Awassi sheep male wool during the breeding season on LH and Progesterone concentrations in Awassi ewes in SyriaHasan Harba1*, Mohamad Moussa1, Abdulmounem Al yasin2, Moataz Zarkawi3 and Lamia Briand Amirat41Department of Surgery and Obstetrics, College of Veterinary Medicine, Hama University, Hama, Syria 2The Arab Center for the Studies of Arid Zones and Dry Lands (ACSAD), Daraa, Syria 3Department of Agriculture, Atomic Energy Commission, Damascus, Syria 4Oniris, Nantes, France *Corresponding Author: Hasan Harba. Department of Surgery and Obstetrics, College of Veterinary Medicine, Hama University, Hama, Syria. Email: hasanharba19 [at] gmail.com Submitted: 15/05/2024 Accepted: 15/09/2024 Published: 31/10/2024 © 2024 Open Veterinary Journal
AbstractBackground: The efficiency and profitability of animal farming systems are closely linked to the reproductive success of livestock, which requires effective management through biological, hormonal, and nutritional strategies. The “male effect,” referring to the influence of male pheromones on female reproductive hormones, offers several benefits. This leads to improved reproductive management, better timing of breeding, and increased fertility rates. Additionally, using natural pheromones provides a non-invasive and sustainable method for managing reproduction, and it can be a cost-effective strategy, reducing the need for expensive hormonal treatments. Incorporating male pheromones into reproductive management practices can thus boost the efficiency and profitability of animal farming systems. Aim: The study’s objective is to examine the influence of pheromones, extracted from Awassi ram’s wool and Shami goat buck’s hair, on the secretion of luteinizing hormone (LH) and Progesterone (P4) in Awassi ewes during the breeding season. Methods: The pheromones were extracted using a soaking method with dichloromethane as the solvent. The resulting organic extract was then analyzed using a Chromatec-Crystal 5,000 gas chromatography-mass spectrometry device, equipped with a quadrupole mass spectrometer. The experiment was carried out on three equal groups, each consisting of 17 subjects, during the breeding season. In Group I, 15 ml of the ram’s wool extract was applied to a piece of gauze and placed in a specially designed mask that was attached directly to the nose. In the second group, a similar procedure was followed using 15 ml of the buck’s hair extract. The third group consisted of Awassi rams. The LH assay was conducted immediately before the treatment and again at 1, 10, and 20 hours post-treatment, and after 5 and 30 days of treatment to assess P4 levels. Results: The study found that pheromones from Awassi rams and Shami goat bucks significantly increased the levels of LH and P4 in female Awassi sheep. Conclusion: The study concludes that the pheromones extracted from the wool of rams and Shami goat bucks can stimulate the response of female Awassi sheep. This finding opens up the possibility of using these pheromones in the reproductive management of female Awassi sheep. Keywords: Awassi sheep, Shami goats, Pheromones, LH, Progesterone. IntroductionThe efficiency and profitability of animal farming systems depend on the reproductive success of livestock. Effective reproductive management involves a combination of biological, hormonal, and nutritional strategies, as well as the prevention of sexually transmitted diseases (Hashem and Gonzalez-Bulnes, 2021). Over time, techniques have been developed to enhance the reproductive efficiency of sheep, such as shortening the non-breeding season and synchronizing lambing periods to specific times of the year (Zarkawi, 2011). This synchronization allows for optimal care conditions, helping farmers distribute labor and feed resources effectively, and ensuring a steady supply of animal products like meat to the market (Godfrey et al., 1997). Various methods have been established to manage the reproduction of farm animals and improve their reproductive performance. Estrus induction and synchronization techniques generally fall into two main categories: hormonal (medical-pharmacological) methods and other key methods related to the care system, such as lighting systems and the male effect. However, hormonal therapies are expensive and have negative impacts such as immunogenic effects. Therefore, using non-invasive and natural methods is crucial for better outcomes. The male effect, recognized as a clean and ethical reproductive control method (Martin et al., 2004; Martin and Kadokawa, 2006), has been known since the 1940s (Ungerfeld et al., 2004). It involves exposing novel males to ewes, as documented by many researchers (Alvarez et al., 2009; Delgadillo et al., 2011). Introducing a sexually active male to a goat herd stimulates female reproductive activity (Avdi et al., 2004). This bio-sexual stimulus, enhanced by numerous male-female sensory contacts, can stimulate or synchronize female reproductive activity even during non-estrus periods, providing a more effective response when direct contact between the sexes occurs (Sampaio et al., 2012). This response primarily depends on ewe olfactory signals produced by male pheromones through androgen stimulation (Gelez and Fabre-Nys, 2004). Pheromones, complex organic molecules, transmit signals from one animal to another and are more specialized than odors, allowing detection in very small quantities. They are secreted by special glands located in specific areas of the animal’s body (such as feet), affecting the receiving individual through their central nervous system and causing behavioral and endocrine changes (Rekwot et al., 2001). After females detect the male’s presence, signals are sent to the central nucleus of the amygdala. This will transfer the signal to the hypothalamus, the origin of gonadotropin-releasing hormone (GnRH) pulses, resulting in a changed pulse frequency of luteinizing hormone (LH) secretion, raising the number of pulses to 2–3 times (Chemineau et al., 1986). Awassi sheep, a prevalent breed in Syria and the Middle East, exhibit seasonal breeding patterns characterized by multiple estrus cycles (Zarkawi et al., 1999). Considering the economic situation in these areas, meeting the growing demand for affordable animal protein is crucial. Sheep are a key source of animal protein and play an essential role in food security. To boost sheep productivity, it is important to enhance reproductive efficiency by developing and implementing new methods focused on increasing productivity. Breeding-based methods are more cost-effective than traditional hormonal approaches, and reproductive traits in sheep can be improved through genetic selection and better breeding practices. Emphasizing natural and sustainable methods, such as breeding and the male effect, can significantly enhance sheep productivity. The purpose of this study was to investigate the impact of pheromones extracted from the wool of Awassi rams and Shami goat bucks' hair on the secretion of LH and progesterone (P4) in Awassi sheep during the breeding season. Material and MethodsPheromone extractionIt was carried out using the soaking method with dichloromethane as the organic solvent. A quantity of 50 g of wool or hair was placed in a 1,000 ml glass container, to which 200 ml of dichloromethane solvent was added. The container was then placed in a vibrating incubator (JSOS-500 JSR) set at 3 g and a temperature of 25°C for a duration of 1 hour. The dichloromethane solvent was replaced twice with the same quantity (200 ml of dichloromethane solvent). The extract was then filtered using filter paper, after which it was concentrated using a rotary evaporator. The final extract was stored in a freezer until required for use. The amount of extraction was calculated using the following formula:
Analysis of organic extractAccording to Tsikolia et al. (2022), The organic extract was examined using a Chromatec-Crystal 5,000 gas chromatography–mass spectrometry system, which is equipped with a quadruple mass spectrometer. The column used was BP-5MS (5% Phenyl Polysilphenylene-siloxane), with dimensions of 30 m × 250 µm and a thickness of 0.25 µm. The temperatures set were as follows: injector at 300°C, ion source at 280°C, selective mass detector at 150°C, and interface at 300°C. The carrier gas used was helium with a purity of 99.9999%. The flow velocity was set at 30.000 cm/second. The syringe volume was 1.0 µl of oil diluted in n-hexane (1/100; V/V). The split ratio was 1:25. The oven temperature was initially set at 50°C for 5.5 minutes, then increased at a rate of 10°C/minute to 290°C, and finally held at 300°C for 5 minutes (Post Run). The total duration of the analysis was 35.50 minutes. The chemical compounds of the extract were identified by comparing their mass spectra with a standard sample in the analyzer library (National Institute of Standards and Technology; NIST). The retention index (RI) of all compounds was determined using the Kovats method, with a homologous series of n-alkanes C8–C22 as the standard. These were then compared with the standards reported in the literature. The RI was computed using:
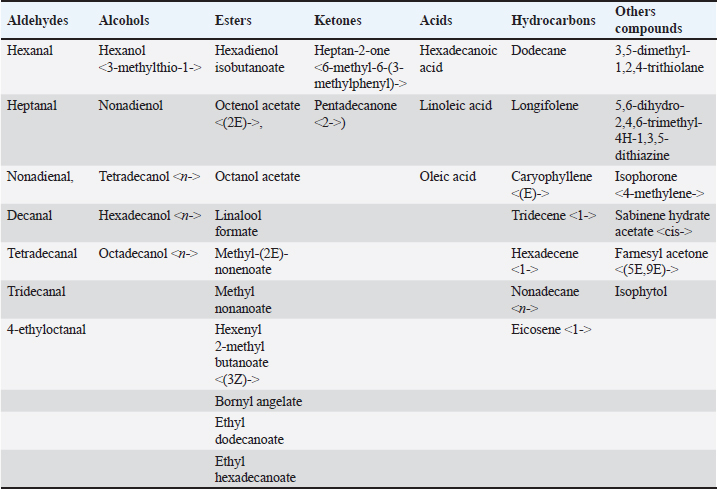
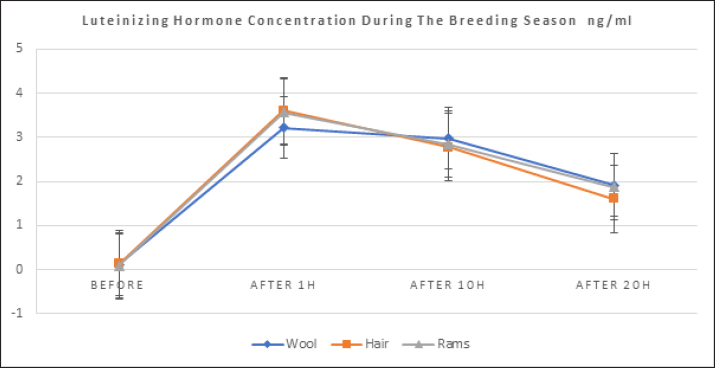
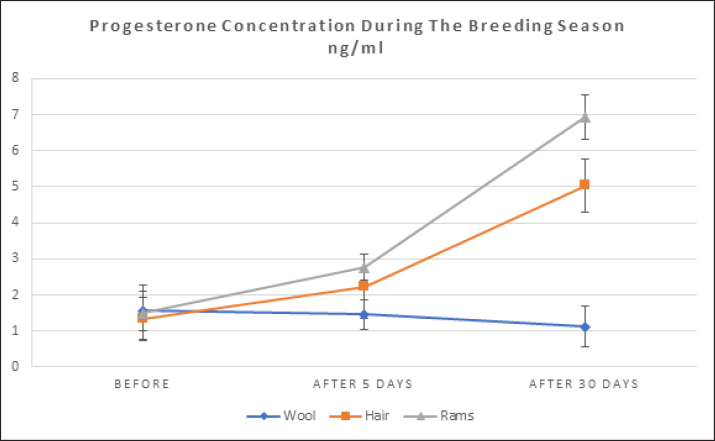
where n: the number of the preceding carbon atom of the sample in a mixture of n-alkanes, N: the number of the following carbon atom of the sample in a mixture of n-alkanes, and RT: retention time. Implementation of the experimentThe experiment was conducted on female Awassi sheep at the Izraa–ACSAD research station. The sheep were aged between 3 and 5 years and had an average weight of 43 ± 2 kg. They were provided with appropriate care, nutrition, and preventive immunizations. The Awassi ewes used in the experiment were isolated from the rest of the animals and completely separated from rams for at least 3 weeks prior to the start of the experiment. The female sheep were randomly divided into three equal groups (n=17/group). The experiments were designed as follows: During the breeding season from 3/7/2022–15/9/2022: i. The first group (Awassi rams wool extract): (n=17): An extract of male sheep wool (15 ml) was applied to a piece of gauze, which was then placed inside a specially designed mask and secured directly on the ewe’ nose. This procedure was carried out for 4 days, half an hour each morning. ii. The second group (Shami goat hair extract): (n=17): Shami goat hair extract (15 ml) was applied to a piece of gauze, which was then placed inside a specially designed mask and secured directly on the ewe’ nose. This procedure was carried out for 4 days, half an hour each morning. iii. The third group (Awassi rams): (n=17) involved the introduction of three males to the females after a 3-week isolation period. • Hormonal assessment: Blood samples were randomly collected from five ewes, and the same ewes were later re-sampled to assess both LH using (Biosite Sheep LH ELISA KIT) and P4 using (Reagent P4 for Biomerieux Vidas–60 Test). A volume of 5 ml of blood was drawn from the animals’ jugular vein and placed in vacuum tubes containing heparin. The sampling was done as follows: i. Just before the start of the treatment assess LH and P4 levels. ii. After initiating the treatment at 1, 10, and 20 hours to assess LH. iii. After 5 and 30 days of treatment to assess P4 levels. The blood samples were centrifuged at 1,008 g for 20 minutes, and the plasma was transferred to specially sealed tubes (two tubes per sample) and stored at −20°C until analysis. Statistical analysisThe statistical study was conducted by making five replicates per group using SPSS version 22 program, using the ANOVA repeated measures test to study the concentrations of (LH and P4 during the breeding season), and the value of the least significant difference (LSD) was calculated at the level of p < 0.05 when there were significant differences between the averages. Ethical approvalThe ethical approval was provided by College of Veterinary Medicine, Hama University, Hama, Syria. ResultsAnalysis of the extractsAnalysis of the extracts indicated that they contain many chemical compounds summarized in Table 1. Luteinizing hormoneFigure 1 shows the rates of increase of the hormone LH within the experimental groups during the breeding season, and it is noted that there was a direct and clear significant increase in the level of the hormone LH after the start of the experiment in females in all groups, which were isolated from males for more than 3 weeks. The results showed significant differences between the mean concentrations of hormones in all groups measured p=0.000 (LSD value=0.63). ProgesteroneFigure 2 shows the levels of P4 hormone within the trial groups during the breeding season, the results showed that there were no significant differences between the average concentrations of P4 between groups (p=0.63), and the results proved that there were significant differences between the average concentrations of P4 between groups 5 days after treatment p =0.000 (LSD value=0.564). The results also showed significant differences between the average concentrations of (P4) between groups 30 days after treatment p=0.000 (LSD value=0.4). DiscussionThe results align with previous studies indicating that when female sheep and goats are exposed to sexually active males during estrus periods, there is an increase in the secretion of LH, which is responsible for ovulation. This immediate response leads to a higher frequency of LH pulses, and concentrations significantly increase after exposure to males, gradually increasing over time (Martin et al., 1980). Alvarez et al. (2009) discovered that female goats exposed to male influence exhibited peak LH between 80 and 317 minutes with 5.7 and 8 pulses and ovulation occurring 8 days after the introduction of the male (Alvarez et al., 2009). The research findings are in line with those of Martin et al. (1980), and Martin et al. (1986), who suggested that the initial endocrine response in the ewe following the introduction of the ram was an increase in LH secretion within 2–4 minutes (Martin et al., 1980). Therefore, exposing females to sexually active males triggers a rapid activation of LH secretion, which reduces the negative feedback mechanism of estradiol on the hypothalamic-pituitary axis and results in pre-ovulation LH secretion (Signoret, 1980). This generates an immediate response (short-term response) followed by a sustained response (long-term response) when this reaction continues (Chanvallon et al., 2010). In terms of P4, the results align with Ungerfeld et al. (2003), who observed a notable rise in P4 production in estrus ewes following the introduction of rams. The findings also concur with Mahmoud and Hussein (2019), who reported that the group where the male effect was combined with prostaglandin (PG) administration exhibited higher P4 concentrations compared to the group that relied solely on PG. The research outcomes are also in line with Godfrey et al. (1999), who demonstrated that P4 levels fell to less than 1 ng/ml during the estrus period and decreased further following the second injection in the PGF2α program (Ganaie et al., 2009). The rise in P4 levels can be attributed to the fact that introducing males led to an increase in the secretion of both LH and FSH, which subsequently resulted in an increase in follicle size and the formation of the corpus luteum (CL), thereby boosting P4 production (Ferreira-Silva et al., 2018). Additionally, some studies have shown a correlation between the production and quantity of the P4 hormone and the number and integrity of granulocytes (Niswender et al., 2000; Niswender, 2002). Table 1. Chemical compounds in hair and wool extract.
Fig. 1. Shows the rates of increase in LH among the experimental groups during the breeding season.
Fig. 2. Shows the rates of increase in P4 among the experimental groups during the breeding season. Numerous releaser pheromones have been identified in mammals (Schaal et al., 2003). For sheep and goats, exposure to male odors can transition females from a seasonally non-reproductive endocrine state to a reproductive one (Martin et al., 1986; Chemineau, 1987). This phenomenon, commonly referred to as the “male effect”, is a notable instance of primer pheromone influence in mammals (Delgadillo et al., 2011; Murata et al., 2009). The ram effect is primarily linked to olfactory signals, presumably pheromones, produced by the male (Claus et al., 2001). Murata discovered a scent molecule that stimulates the GnRH pulse generator, a crucial reproductive regulator in goats (Murata et al., 2014). Through gas chromatography-mass spectrometry analysis of volatile substances from male goats, they identified various ethyl-branched aldehydes and ketones. Their research electro-physiologically verified that 4-ethyloctanal, one of these substances, activates the GnRH pulse generator in female goats. This compound is also a vital component of the male goat scent, significantly influencing female attraction. Moreover, when 4-ethyloctanal undergoes oxidation, it converts into 4-ethyloctanoic acid, the primary component responsible for the distinctive goaty aroma (Murata et al., 2014). It is noteworthy that the chemical compounds 4-methyl octanoic acid and 4-ethyl octanoic acid, present in the pheromones emitted by male goats and sheep, exhibit a significant similarity. These compounds, found in varying ratios, are believed to contribute to the “male effect”. This similarity provides an explanation for the findings reported by (Claus et al., 2001). Our findings on the impact of pheromones contrast with a study that reported no changes in LH or FSH secretion (Schneider and Rehbock, 2003). However, other studies have found that pheromone use led to ovulation (Kaulfulb et al., 1997, 2002) or increased pregnancy rates in inseminated ewes. It has also been observed that full contact is not necessary for a response between ewes and rams (Watson and Radford, 1960) and that the scent of wool and wax from healthy rams is sufficient to elicit an ovulation response in ewes (Knight and Lynch,1980). Morgan noted that ewes with a weak scent did not respond to rams (Morgan et al., 1972), but a normal LH response was observed in ewes reported as lacking olfactory activity (Cohen-Tannoudji et al., 1989). Researchers have indicated that both wool and wax are the primary sources of pheromones that contribute to the ram effect (Knight and Lynch, 1980), and that pheromones produced by males can also induce pulsed frequency secretion of LH (Over et al., 1990) and ovulation in diestrus ewes (Knight et al., 1983). Pheromones have been found in aqueous extracts of wool and wax (Knight and Lynch, 1980), and are produced by the skin, particularly around the eyes (Martin, 2001). The concentration of extracted pheromones, the extraction method, and their application to animals can contribute to the variation in results between studies. However, we can say the male effect enhances reproductive efficiency in female sheep and goats by transitioning them from a non-reproductive to a reproductive state through male pheromones. The scent of wool and wax from healthy rams can trigger ovulation even without direct contact. Pheromones from wool, wax, and skin, especially around the eyes, play a crucial role in this process, making it a natural, efficient, and effective method for improving reproductive outcomes in livestock. ConclusionExposing female sheep and goats to sexually active males during estrus periods significantly increases LH secretion, which is crucial for ovulation. This response includes both immediate and sustained increases in LH pulses. The "male effect," driven by male pheromones, transitions females from a non-reproductive to a reproductive state. Pheromones from wool and wax are primary contributors to this effect, and full contact between ewes and rams is not necessary for a response. However, variations in results between studies may be due to differences in pheromone concentration, extraction methods, and application techniques. AcknowledgmentsNone. Conflict of interestThe authors declare that there is no conflict of interest. FundingACSAD (Arab Center for Studies of Arid and Dry Lands) is considered the primary supporter in conducting the research, as the experiment was implemented on its farms, while the Atomic Energy Commission provided the laboratories in which the hormonal analyzes were conducted and (GCMS) device was used, and the Oniris Institute in France provided Hormonal Kits, and pheromone extraction was performed at the Faculty of Science at the University of Damascus in Syria. The University of Hama in Syria also partially contributed to the financial costs. Authors’ contributionsHassan Harba is the primary researcher, implementer, and writer of the research, as this research is part of the doctoral thesis that is currently being prepared. Dr. Mohamad Moussa supervises the research as a scientific supervisor and assistant in directing and writing the thesis, and Dr. Abdel Moneim AlYasin is the assistant practical supervisor during the implementation of the research in the ACSAD organization. Dr. Moataz Zarkawi is the co-supervisor from the Atomic Energy Commission, where he helped conduct tests and analyzes related to the research plan, and Dr. Lamia Briand Amirat played a role in securing hormonal analysis kits and helping to direct and revise some aspects of the thesis. Data availabilityAll data are provided in the manuscript. ReferencesAlvarez, L., Ramos, A.L. and Zarco, L. 2009. The ovulatory and LH responses to the male effect in dominant and subordinate goats. Small. Rumin. Res. 83, 29–33. Avdi, M., Leboeuf, B. and Terqui, M. 2004. Advanced breeding and “buck effect” in indigenous Greek goats. Livest. Prod. Sci. 87, 251–257. Chanvallon, A., Scaramuzzi, R.J. and Claude, F.N. 2010. Early sexual experience and stressful conditions affect the response of young ewes to the male. Physiol. Behav. 99, 457–465. Chemineau, P. 1987. Possibilities for using bucks to stimulate ovarian and oestrous cycles in anovulatory goats-a review. Livest. Prod. Sci. 17, 135–147. Chemineau, P., Normant, E., Ravault, J.P. and Thimonier, J. 1986. Induction and persistence of pituitary and ovarian activity in the out-of-season lactating dairy goat after a treatment combining a skeleton photoperiod, melatonin and the male effect. Reproduction 78, 497–504. Claus, R., Martin, D., Ursula, G. and Markus, L. 2001. The pheromone of the male goat: function, sources, androgen dependency and partial chemical characterization. Chem. Signal. Verteb. 9, 133–140. Cohen-Tannoudji, J., Lavenet, C., Aude, L., Yves, T. and Signoret, J.P. 1989. Non-involvement of the accessory olfactory system in the LH response of anoestrous ewes to male odour. Reproduction 86, 135–144. Delgadillo, J.A., Ungerfeld, R., Flores, J.A., Hernandez, H. and Fitz-Rodríguez, G. 2011. The ovulatory response of anoestrous goats exposed to the male effect in the subtropics is unrelated to their follicular diameter at male exposure. Reprod. Domest. Anim. 46, 687–691. Ferreira-Silva, J.C., Fernando, T.F., Marcelo, T.M., Pábola, S.N., Luis, R.S.O., Claudio, C.B. and Marcos, A.L.O. 2018. Follicular size, luteinizing hormone (LH), and progesterone (P4) levels in postpartum Santa Inês ewes subjected to ram effect combined with suckling interruption. Livest. Prod. Sci. 214, 88–92. Ganaie, B.A., Khan, M.Z., Quresh, S., Islam, R. and Wani, G.M. 2009. Plasma progesterone profile during gestation and peripartum period in Corriedale sheep. Indian J. Anim. Reprod. 30, 18–21. Gelez, H. and Fabre-Nys, C. 2004. The "male effect" in sheep and goats: a review of the respective roles of the two olfactory systems. Horm. Behav. 46(3), 257–271. Godfrey, R.W., Collins, J.R., Hensley, E.L. and Wheaton, J.E. 1999. Estrus synchronization and artificial insemination of hair sheep ewes in the tropics. Theriogenology 51, 985–997. Godfrey, R.W., Gray, M.L. and Collins, J.R. 1997. A comparison of two methods of oestrous synchronisation of hair sheep in the tropics. Anim. Reprod. Sci. 47, 99–106. Hashem, N.M. and Gonzalez-Bulnes, A. 2021. Nanotechnology and reproductive management of farm animals: challenges and advances. Animals 11, 1932. Kaulfulb, K.H., Schenk, P. and Süb, R. 2002. Die Brunstinduktion saisonal anostrischer Schafe durch nasale applikation von pheromonhaltigem Schafbockwollfett. [Estrus induction of seasonally anestrous ewes by nasal application of ram pheromone containing wool fat]. Tierarztliche Praxis 30, 308–314. Kaulfulb, K.H., Süb, R., Rummer, K., Prange, H. and Borell, E.V. Ovarian reaction after pheromone application in anoestrous German Mutton Merino ewes in relation to ovary state before stimulation. In 48th Annual Meeting of the European Association of Animal Production, Vienna, Austria, 1997. Knight, T.W. and Lynch, P.R. 1980. Source of ram pheromones that stimulate ovulation in the ewe. Anim. Reprod. Sci. 3, 133–136. Knight, T.W., Tervit, H.R. and Lynch, P.R. 1983. Effects of boar pheromones, ram's wool and presence of bucks on ovarian activity in anovular ewes early in the breeding season. Anim. Reprod. Sci. 6, 129–134. Mahmoud, G.B. and Hussein, H.A. 2019. Ram effect on estrus behavior, ovarian structure and steroid hormone levels in Ossimi ewes treated with prostaglandin F2α for estrus synchronization. Egypt. J. Anim. Prod. 56, 87–92. Martin, G.B. 2001. Role of pheromones in wild and domesticated mammals. Adv. Ethol. 36, 29. Martin, G.B., Milton, J.T.B., Davidson, R.H., Banchero Hunzicker, G.E., Lindsay, D.R. and Blache, D. 2004. Natural methods for increasing reproductive efficiency in small ruminants. Anim. Reprod. Sci. 82, 231–245. Martin, G.B., Oldham, C.M. and Lindsay, D.R. 1980. Increased plasma LH levels in seasonally anovular Merino ewes following the introduction of rams. Anim. Reprod. Sci. 3, 125–132. Martin, G.B. and Kadokawa, H. 2006. "Clean, green and ethical" animal production. Case study: reproductive efficiency in small ruminants. J. Reprod. Dev. 52, 145–152. Martin, G.B, Oldham, C.M., Cognié, Y. and Pearce, D.T. 1986. The physiological responses of anovulatory ewes to the introduction of rams—a review. Livest. Prod. Sci. 15, 219–247. Morgan, P.D., Arnold, G.W. and Lindsay, D.R. 1972. A note on the mating behaviour of ewes with various senses impaired. Reproduction 30, 151–152. Murata, K., Shigeyuki, T., Masamichi, I., Yasutaka, O., Yoshihiro, W., Hidenori, W., Hiroaki, O., Yukari, T. and Yuji, M. 2014. Identification of an olfactory signal molecule that activates the central regulator of reproduction in goats. Curr. Biol. 24, 681–686. Murata, K., Wakabayashi, Y., Kitago, M., Ohara, H., Watanabe, H., Tamogami, S., Warita, Y., Yamagishi, K., Ichikawa, M. and Takeuchi, Y. 2009. Modulation of gonadotrophin-releasing hormone pulse generator activity by the pheromone in small ruminants. J. Neuroendocrinol. 21, 346–350. Niswender, G.D. 2002. Molecular control of luteal secretion of progesterone. Reproduction 123, 333–339. Niswender, G.D., Juengel, J.L., Silva, P.J., Rollyson, M.K. and McIntush, E.W. 2000. Mechanisms controlling the function and life span of the corpus luteum. Physiol. Rev. 80, 1–29. Over, R., Cohen-Tannoudji, J., Dehnhard, M., Claus, R. and Signoret, J.P. 1990. Effect of pheromones from male goats on LH-secretion in anoestrous ewes. Physiol. Behav. 48(5), 665–668. Rekwot, P.I., Ogwu, D., Oyedipe, E.O. and Sekoni, V.O. 2001. The role of pheromones and biostimulation in animal reproduction. Anim. Reprod. Sci. 65, 157–170. Sampaio, J.A.R., Maria, G.F.S., Camilo, A.T. and Airton, A.A. 2012. Efeito macho interespécie: indução de estro em cabras leiteiras pela presença de macho ovino. Rev. Bras. Hig. Sanid. Anim. 6, 51–64. Schaal, B., Gérard, C., Dominique, L., Christian, G., Etienne, S. and Guy, P. 2003. Chemical and behavioural characterization of the rabbit mammary pheromone. Nature 424, 68–72. Schneider, F. and Rehbock, F. 2003. Induction of fertile cycles in the Blackhead sheep during the anoestrus period. Arch. Anim. Breed. 46, 47–61. Signoret, J.P. 1980. Effect of the male presence on the reproductive mechanisms in female mammals. Reprod. Nutr. Dev. 20, 457–468. Tsikolia, M., Tabanca, N., Kline, D.L., Demirci, B., Yang, L., Linthicum, K.J., Bloomquist, J.R. and Bernier, U.R. 2022. Studies on the volatiles composition of stored sheep wool, and attractancy toward Aedes aegypti mosquitoes. Insects 13, 208. Ungerfeld, R., Forsberg, M. and Rubianes, E. 2004. Overview of the response of anoestrous ewes to the ram effect. Reprod. Fertil. Dev. 16, 479–490. Ungerfeld, R., Suárez, G., Carbajal, B., Silva, L., Laca, M., Forsberg, M. and Rubianes, E. 2003. Medroxyprogesterone priming and response to the ram effect in Corriedale ewes during the nonbreeding season. Theriogenology 60, 35–45. Watson, R.H. and Radford, H.M. 1960. The influence of rams on onset of oestrus in Merino ewes in the spring. Aust. J. Agric. Res. 11, 65–71. Zarkawi, M. 2011. Response of fat-tailed Syrian Awassi ewes to accelerated lambing systems. Trop. Anim. Health Prod. 43, 1311–1318. Zarkawi, M., Al-Merestani, M.R. and Wardeh, M.F. 1999. Induction of synchronized oestrous and early pregnancy diagnosis in Syrian Awassi ewes, outside the breeding season. Small Rumin. Res. 33, 99–102. | ||
| How to Cite this Article |
| Pubmed Style Harba H, Moussa M, Alyasin AM, Zarkawi M, Amirat LB. The application of pheromones extracted from Shami goats' male hair and Awassi sheep male wool during the breeding season on LH and Progesterone concentrations in Awassi ewes in Syria. Open Vet. J.. 2024; 14(10): 2564-2571. doi:10.5455/OVJ.2024.v14.i10.6 Web Style Harba H, Moussa M, Alyasin AM, Zarkawi M, Amirat LB. The application of pheromones extracted from Shami goats' male hair and Awassi sheep male wool during the breeding season on LH and Progesterone concentrations in Awassi ewes in Syria. https://www.openveterinaryjournal.com/?mno=201574 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i10.6 AMA (American Medical Association) Style Harba H, Moussa M, Alyasin AM, Zarkawi M, Amirat LB. The application of pheromones extracted from Shami goats' male hair and Awassi sheep male wool during the breeding season on LH and Progesterone concentrations in Awassi ewes in Syria. Open Vet. J.. 2024; 14(10): 2564-2571. doi:10.5455/OVJ.2024.v14.i10.6 Vancouver/ICMJE Style Harba H, Moussa M, Alyasin AM, Zarkawi M, Amirat LB. The application of pheromones extracted from Shami goats' male hair and Awassi sheep male wool during the breeding season on LH and Progesterone concentrations in Awassi ewes in Syria. Open Vet. J.. (2024), [cited January 25, 2026]; 14(10): 2564-2571. doi:10.5455/OVJ.2024.v14.i10.6 Harvard Style Harba, H., Moussa, . M., Alyasin, . A. M., Zarkawi, . M. & Amirat, . L. B. (2024) The application of pheromones extracted from Shami goats' male hair and Awassi sheep male wool during the breeding season on LH and Progesterone concentrations in Awassi ewes in Syria. Open Vet. J., 14 (10), 2564-2571. doi:10.5455/OVJ.2024.v14.i10.6 Turabian Style Harba, Hasan, Mohamad Moussa, Abdel Moneim Alyasin, Moataz Zarkawi, and Lamia Briand Amirat. 2024. The application of pheromones extracted from Shami goats' male hair and Awassi sheep male wool during the breeding season on LH and Progesterone concentrations in Awassi ewes in Syria. Open Veterinary Journal, 14 (10), 2564-2571. doi:10.5455/OVJ.2024.v14.i10.6 Chicago Style Harba, Hasan, Mohamad Moussa, Abdel Moneim Alyasin, Moataz Zarkawi, and Lamia Briand Amirat. "The application of pheromones extracted from Shami goats' male hair and Awassi sheep male wool during the breeding season on LH and Progesterone concentrations in Awassi ewes in Syria." Open Veterinary Journal 14 (2024), 2564-2571. doi:10.5455/OVJ.2024.v14.i10.6 MLA (The Modern Language Association) Style Harba, Hasan, Mohamad Moussa, Abdel Moneim Alyasin, Moataz Zarkawi, and Lamia Briand Amirat. "The application of pheromones extracted from Shami goats' male hair and Awassi sheep male wool during the breeding season on LH and Progesterone concentrations in Awassi ewes in Syria." Open Veterinary Journal 14.10 (2024), 2564-2571. Print. doi:10.5455/OVJ.2024.v14.i10.6 APA (American Psychological Association) Style Harba, H., Moussa, . M., Alyasin, . A. M., Zarkawi, . M. & Amirat, . L. B. (2024) The application of pheromones extracted from Shami goats' male hair and Awassi sheep male wool during the breeding season on LH and Progesterone concentrations in Awassi ewes in Syria. Open Veterinary Journal, 14 (10), 2564-2571. doi:10.5455/OVJ.2024.v14.i10.6 |