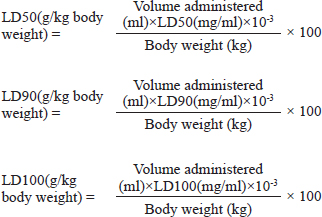| Short Communication | ||
Open Vet. J.. 2021; 11(4): 651-661 Open Veterinary Journal, (2021), Vol. 11(4): 651–661 Short Communication Galleria mellonella (greater wax moth) as an eco-friendly in vivo approach for the assessment of the acute toxicity of medicinal plants: Application to some plants from CameroonMbarga Manga Joseph Arsene*, Podoprigora Irina Viktorovna and Anyutoulou Kitio Linda DavaresDepartment of Microbiology and Virology, Institute of Medicine, RUDN University, Moscow, Russia *Corresponding Author: Mbarga Manga Joseph Arsene. Department of Microbiology and Virology, Institute of Medicine, RUDN University, Moscow, Russia. Email: josepharsenembarga [at] yahoo.fr Submitted: 22/07/2021 Accepted: 28/10/2021 Published: 15/11/2021 © 2021 Open Veterinary Journal
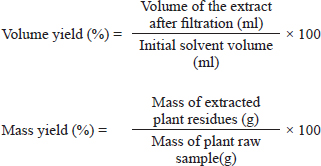
AbstractBackground: The evaluation of medicinal plants’ toxicity is a prerequisite prior to their usage. The vertebrate models used for this purpose are often the object of ethical consideration. Though invertebrate models including Galleria mellonella (GM) have demonstrated the ability to be used to assess the toxicity of various products. To the authors’ knowledge, GM has never been exploited to determine the toxicity of medicinal plants. Aim: The aim of this study was to demonstrate the potential of GM larvae as a simple, inexpensive, and rapid model for the evaluation of the toxicity of herbal medicines. Method: In this study, the toxicity of aqueous and ethanolic (80%, v/v) extracts of seven well known plants from Cameroon namely leaves of Cymbopogon citratus (DC.) Stapf, Moringa oleifera Lam and Vernonia amygdalina Delile; barks of Cinchona officinalis and Enantia chlorantha Oliv; barks and seeds of Garcinia lucida Vesque and leaves and seeds of Azadirachta indica (Neem) was evaluated using the larval form of GM. The median lethal doses (LD50), 90% (LD90), and 100% (LD100) were determined using the spline cubic survival curves and equations from the data obtained on the survival rate of GM 24 hours after the injection with the extracts. Results: We found that distilled water extracted a more important mass of phytochemical compounds (7.4%–21.2%) compared to ethanolic solution (5.8%–12.4%). LD varied depending on the plant materials and ethanolic extracts (hydroalcoholic extract, (HAE)) were more toxic to GM than aqueous ones. The LD50 (mg/ml) of the tested extracts varied from 4.87 [3.90 g/kg body weight (bw)] to >200 (> 166.67 g/kg bw), the LD90 (mg/ml) from 25.00 (18.52 g/kg bw) to >200 (> 181.82 g/kg bw) and LD100 (mg/ml) from 45.00 (40.91 g/kg bw) to > 200 (>181.82 g/kg bw). The HAE of A. indica seed and C. officinalis bark exhibited the highest toxicity with LD50 (g/kg bw) of 3.90 and 4.81, respectively. Conclusion: The results obtained in this study suggest that GM can be used as a sensitive, reliable, and robust eco-friendly model to gauge the toxicity of medicinal plants. Thus, avoid the sacrifice of vertebrate models often used for this purpose to limit ethical concerns. Keywords: Medicinal plants, Greater wax moth, Galleria mellonella, Insect model, Toxicity. IntroductionMedicinal plants have been used for millennia to treat various maladies including respiratory tract infections, urinary tract infections, sepsis, diabetes, erectile dysfunction, diarrhea, fevers and as a stabilizer of physiological functions among many others (Kuete et al., 2010; Sylvie et al., 2014). In this study, the herbal medicines used were leaves of Cymbopogon citratus (DC.) Stapf, Moringa oleifera Lam, and Vernonia amygdalina Delile; barks of Cinchona officinalis and Enantia chlorantha Oliv; barks and seeds of Garcinia lucida Vesque and Leaves and seeds of Azadirachta indica (Neem). These medicinal plants are among the most popular in Cameroon (Kuete et al., 2010; Sylvie et al., 2014; Sonfack et al., 2021) Cinchona officinalis is a shrub of the Rubiaceae family whose bark is recognized for its very bitter taste and its composition in alkaloids, such as quinine, dihydroquinine, cinchonidine, epiquinine, quinidine, dihydroquinidine, cinchonine, and epiquinidine (Júnior et al., 2012; Bharadwaj et al., 2018). In addition to its antimalarial properties, C. officinalis has also been used on a smaller scale as a treatment for goiter, Meniere’s disease (an inner ear disorder), varicose veins, and in obstetrics for its action on uterine muscles (Júnior et al., 2012). Garcinia lucida Vesque (G. Lucida) is also a well-known herbal medicine whose properties and composition have been well-studied. The seed, fruit, and bark are the most commonly used parts in traditional medicine and food (Sylvie et al., 2014). Used in fermentation of palm tree or raffia wine (Guedje et al., 2017), the bark and seeds have been found useful in the treatment of gastric and gynecological infections, diarrheas, cure for snake bites as well as an antidote against poison (Sylvie et al., 2014). In addition, a recent study conducted by Sonfack et al. (2021) revealed that the aqueous extract from the stem bark of G. lucida exhibits cardioprotective and nephroprotective effects in adenine-induced chronic kidney disease in rats. This plant is also believed to possess some aphrodisiac properties and also used in ghost-hunting practices (Guedje and Fankap, 2001). Investigations on the chemical composition of G. lucida have shown that it contains the terpenoids and xanthones products such as 1,2-dihydroxy-xanthone, 1-hydroxy-2-methoxyxanthone, putranjivic acid, methyl putranjivate, and friedelin (Kuete et al., 2010; Sylvie et al., 2014). Enantia chlorantha Oliv (E. chlorantha) is a plant belonging to the Annonaceae family with multiple uses in traditional medicines. This versatile plant is also referred to as Epoue (Baka), Peye (Badjoue), and Nfol (Bulu). It is widely spread along Sub-Saharan Africa (Etame et al., 2019). In Cameroon, E. chlorantha is used in the management of various infections including malaria, typhoid fever, jaundice, dysentery, wounds, high blood pressure, urinary infection, leprosy spots, and convulsions. Its yellow, dried, or fresh bark has been reported to have antiviral (Ohemu et al., 2018), antifungal (Abike et al., 2020), and antibacterial properties (Etame et al., 2019; Abike et al., 2020). Other studies have reported its use as an antioxidant and antipyretic (Olivier et al., 2015). Investigations into its composition revealed that E. chlorantha contains numerous biologically active compounds, the most important of which are alkaloids such as protoberberines (berberine, canadine, palmatine, jatrorrhizine, columbamine, and pseudocolumbamine), phenanthrene alkaloids (atherosperminine and argentinine), and aporphines (7-hydroxydehydronuciferine and 7-hydroxydehydronornuciferine) (Olivier et al., 2015). Azadirachta indica (A. indica) known as Neem is a monoecious tree of the Meliaceae family whose oil produced from its seeds is widely used for its medicinal properties in the northern part of Cameroon. It is known that compounds in Neem extracts have anti-inflammatory, anti-hyperglycaemic, anti-carcinogenic, antimicrobial, immune-modulator, anti-mutagenic, antioxidant, anti-ulcer, and anti-viral effects (Arévalo-Híjar et al., 2018; Baildya et al., 2021). Recent studies by Baildya et al. (2021) found 19 compounds from this plant which may be used to fight against COVID-19. In addition, Azadirachtin, one of its major constituents, gives it insecticidal properties which acts by intercepting metamorphosis from the larval to adult stage, and paralyzes the digestive tract of the larva (Tofel et al., 2017; Meisyara et al., 2019). The different parts of A. indica have been widely used in recent years in the green synthesis of nanoparticles such as silver nanoparticles (AgNPs), Zinc oxide nanoparticles (ZnONPs), gold (AuNPs) and copper nanoparticles (CuNPs) (Nagar and Devra, 2018; Abbas et al., 2020; Vijayakumar et al., 2020; Chinnasamy et al., 2021). Moringa oleifera Lam (M. oleifera) is both an edible and a medicinal plant. Every part of the plant, from the leaves to the roots, has been reported to possess potential health benefits (Aderinola et al., 2020). This plant belonging to the Moringaceae family contains a profile of important minerals, and is a good source of protein, vitamins, β-carotene, amino acids, and various phenolic compounds (Anwar et al., 2007). Moringa oleifera provides a rich and rare combination of zeatin, quercetin, β-sitosterol, caffeoylquinic acid, and kaempferol (Anwar et al., 2007; Aderinola et al., 2020). Besides its nutritional properties, M. oleifera is traditionally used to treat skin infections, asthma, diabetes, diarrhea, arthritis, inflammation, cough, fever, and headache. It has also been reported to have antioxidant, anti-inflammatory, antitumor, antimicrobial, hepatoprotective, and anti-arthritic properties (Ray et al., 2015; Arulselvan et al., 2016; Saleem et al., 2020). Like M. oleifera, V. amygdalina Delile (V. amygdalina) is both an edible and a medicinal plant. Vernonia amygdalina belongs to the family Asteraceae and is called bitter leaf in English because of its bitter taste. In Cameroon, V. amygdalina is known under the name of Ndolè and is included in the composition of the national popular dish which bears the same name. The presence of polyphenols, vitamins, and mineral salts makes the plant useful in human diets (Tonukari et al., 2015; Dumas et al., 2021). Moreover, this plant is rich in phytochemicals such as saponins, sesquiterpenes, flavonoids, and steroid glycosides (vernosides), lactones (Dumas et al., 2021) and several studies reported that it has anticancer and antitumor activity (Hasibuan et al., 2020; Joseph et al., 2020); antihepatotoxic activity (Yedjou et al., 2018); hypoglycemic activity (Dumas et al., 2021); antibacterial activity (Egbuonu and Amadi, 2021); anti-inflammatory (Wang et al., 2020) as well as antioxidant property (Alara and Abdurahman, 2021). Cymbopogon citratus (DC.) Stapf (C. citratus) or lemongrass is a herbaceous plant of the Poaceae family growing in humid tropical areas which is mainly cultivated for its stems and leaves whose infusion gives a tea with strong lemony odor due to its high content of the aldehyde citral. (Do et al., 2021). Muala et al. (2021) reported that lemongrass is rich in minerals, vitamins, macronutrients (including carbohydrate, protein, and small amounts of fat) and its leaves are a good source of various bioactive compounds such as alkaloids, terpenoids, flavonoids, phenols, saponins, and tannins that confer C. citratus leaves pharmacological properties such as anti-cancer, antihypertensive, anti-mutagenicity, anti-diabetic, antioxidant, anxiolytic, anti-nociceptive, and anti-fungi. Cymbopogon citratus is also commonly used in folk medicine for treatment of nervous and gastrointestinal disturbances, and as an antispasmodic, analgesic, anti-inflammatory, anti-pyretic, diuretic, and a sedative (Hanaa et al., 2012). In general, medicinal plants constitute a source of new biologically active molecules that are economically accessible to deal with the emergence of phenomena of resistance of germs to chemical molecules (Koné et al., 2020). The common point of all these medicinal plants (and all the molecules that result from them) that can potentially be used in the treatment of various pathologies is the evaluation of their toxicity. Indeed, toxicity testing in rodents is an important prerequisite to the use of compounds in man (Ignasiak and Maxwell, 2017). However, trials in rats and mice are expensive, not eco-friendly and there are ethical considerations (Ignasiak and Maxwell, 2017). In this context, invertebrate models appear as a very promising alternative and have seen a gain in popularity in recent years (Megaw et al., 2015; Ignasiak and Maxwell, 2017; Allegra et al., 2018; Wijesinghe et al., 2020; Moya-Andérico et al., 2021). The most used invertebrate models for toxicity trials among others are Artemia salina, Daphnia magna, Drosophila melanogaster, soil invertebrate Folsomia candida, the nematode Caenorhabditis elegans, and the greater wax moth Galleria mellonella (GM) (Megaw et al., 2015; Ogungbemi and van Gestel, 2018; Land et al., 2020; Zhang et al., 2021). Among all these in vivo insect models, GM has a number of attractive benefits due to its practicability, in particular its low cost, ease of acquisition and handling (no specialist training or equipment is required), ability to survive at 37°C (unlike many other non-mammalian hosts), they are large enough (2 cm in length and weigh approximately 250 mg) for accurate dosing (unlike commonly used invertebrate models such as C. elegans and D. melanogaster) which make it possible to handle individual larvae and administer test compounds directly into each individual (which is not always possible with other smaller invertebrate models) and enhances the reproducibility of assays conducted using this model (Megaw et al., 2015; Ignasiak and Maxwell, 2017; Piatek et al., 2020). The aim of this study was to demonstrate the potential of GM larvae as a simple, inexpensive, and rapid model for the evaluation of the toxicity of herbal medicines such as C. officinalis, G. lucida Vesque, E. chlorantha Oliv, A. indica (Neem), M. oleifera Lam, V. amygdalina Delile, and C. citratus. To the authors’ knowledge, this study presents the first use of GM for an in vivo lethal doses (LD50) study of medicinal plants. Material and MethodsPlant materialThe plant material used in this study were leaves of C. citratus (DC.) Stapf, M. oleifera Lam and V. amygdalina Delile; barks of C. officinalis and E. chlorantha Oliv; barks and seeds of G. lucida Vesque and leaves as well as seeds of A. indica (Neem). These plants were chosen because they are renowned for their use in traditional medicine in Cameroon and because of data existing in the literature on their effectiveness and their composition. All the plants were collected in September 2020 in different regions of Cameroon. Vernonia amygdalina and C. citratus was collected in Nlobison II, Central region of Cameroon (VJ5J + C3 Yaounde, Cameroon); C. officinalis, E. chlorantha, and G. lucida were bought in the Nkoabang Market (VH7M+FJ Yaoundé, Cameroun); M. oleifera was bought in the Dang Market of Adamaoua region (CHH5 + 67 Ngaoundéré, Cameroun) and A. indica was bought in the North region (7CX2 + X4 Garoua, Cameroun). The collected plants were dried at room temperature in the shade for 7 days then packaged in hermetically sealed plastics and additional packaging was done to facilitate shipment to Russia in December 2020 where they were received by the Laboratory of Microbiology, Faculty of Medicine of the RUDN University. Plants were grinded and the powders with particle sizes lower than 1 mm were stored in a sterile airtight container until further use. Galleria mellonellaGM larvae were obtained commercially from https://ecobaits.ru/ (ECO BAITS, Moscow, Russia) and stored at 15°C prior to use. Dead larvae and those with dark spots or showing signs of melanization were discarded. Extraction of active compoundsEthanolic solution (80%, v/v) and distilled were used because they have been reported to be efficient solvents for the extraction of bioactive compounds in the medicinal plants used in this study. As we described in our previous investigation (Mbarga et al., 2021), 50 g of plant material was weighed and added to 450 ml of the solvent in separate conical flasks. The flasks were covered tightly and were shaken at 200 rpm for 24 hours and 25°C in a shaker incubator (Heidolph Inkubator 1000 coupled with Heidolph Unimax 1010, Germany). The mixtures were then filtered by vacuum filtration, using Whatman filter paper № 1 then concentrated at 40°C in rotary evaporator (IKA RV8) equipped with a water bath IKA HB10 (IKA Werke, Staufen, Germany) and a vacuum pumping unit IKA MVP10 (IKA Werke, Staufen, Germany). In order to avoid losses, the extracts were collected when the volumes were small enough and placed in Petri dishes previously weighed and then incubated open at 40°C until complete evaporation. The final dried crude extracts were weighed. Extract volume and mass yield were determined using the following formulas:
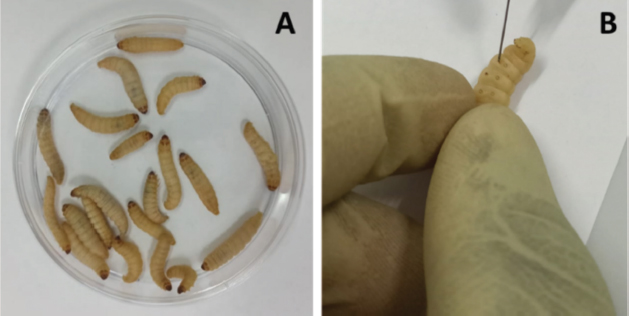
Stock solutionsOne gram of each crude extract was dissolved in 5 ml of sterile phosphate-buffered saline (PBS) to obtain the concentration of 200 mg/ml. These solutions were sterilized by microfiltration (0.22 μm) and dilutions were performed in sterile PBS to obtain concentrations of 150, 100, 50, 25, 12.5, and 5 mg/ml. The sterile PBS plant extract free was used as control. All the eight tests solution were stored at 4°C. Toxicity assayThe larvae were weighed and only those from 0.2 to 0.5 g were retained. For each concentration of extract, 3 groups of 20 randomly selected GM larvae were used (Fig. 1A). 20 μl of each dilution were injected using a 0.3 ml Terumo® Myjector® U-100 insulin syringe (VWR, Russia) through the base of the last left proleg as described by Megaw et al. (2015), and shown in Figure 1B. Control groups of 10 larvae injected with 20 μl sterile PBS were also included. After 24 hours of incubation in the dark at 37°C, larvae were examined for mortality, and were considered dead if they were unmoving, failed to reorient themselves when placed on their backs, and failed to respond to stimuli as indicated by Megaw et al. (2015). Percentage survival was plotted as a function of concentration for each plant extract sample using Spline cubic model in the statistical software XLSTAT 2020 (Addinsof Inc., New York) and median LD50, LD90 and LD100 values expressed in mg/ml were calculated using each specific spline cubic equation or spline cubic curves obtained. Spline curves were used when the fit accuracy of the spline equation was less than 90%). The mean weight of each group of 20 larvae was used to extrapolate the LD50, LD90 and LD100 values for each plant extract sample in g/kg using the formulas:
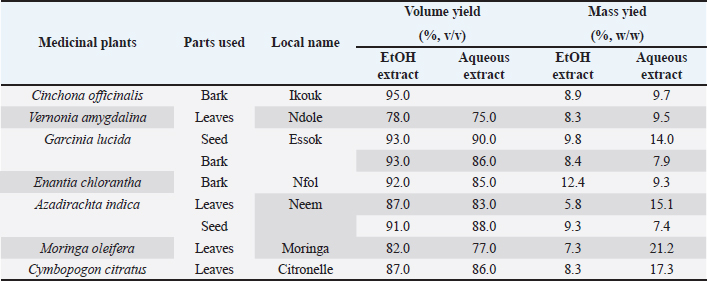
Results and DiscussionThe extract yields obtained for the nine samples from our seven medicinal plants using ethanolic solution and distilled water are recorded in Table 1. As observed in Table 1, the highest volume yields were observed with ethanolic extracts while the highest mass yields were obtained with aqueous leaves extract of M. oleifera (21.2%), C. citratus (17.3%), A. indica (15.1%), and V. amygdalina (14.0%). The highest mass yield with ethanolic solution was obtained with E. chlorantha bark (12.4%). Extraction with ethanolic solution was less effective in A. indica seed and M. oleifera leaves with mass yields of 5.8% and 7.3%, respectively. In most of the studies where extraction of active compounds was performed, it was reported that water had a better extraction rate than other solvents such as ethanol or methanol (Arsene et al., 2021; Mbarga et al., 2021; Mouafo et al., 2021). Mouafo et al. (2021) reported that this difference is mainly attributed to the polarity of the solvents and therefore to the large proportions of water-soluble compounds present in the plant. However, high yields of phytoconstituents obtained does not necessarily imply a high biological activity (Mouafo et al., 2021). We observed this in our study through the (LD50, LD90, and LD100) determined for each of the extracts of the medicinal plants tested. Indeed, as shown in Table 3, LD50, LD90, and LD100 values vary considerably from one plant to another but above all, we noticed that for all medicinal plants, the LD of ethanolic extracts were lower than those of aqueous extracts. This implies that ethanolic extracts contain more active compounds and are therefore more toxic than aqueous extracts because, as reported by Situmorang et al. (2020), low LD50 values are associated with high toxicity (high amount of active compounds) and conversely, high LD50 values are associated with low toxicity (low amount of active compounds).
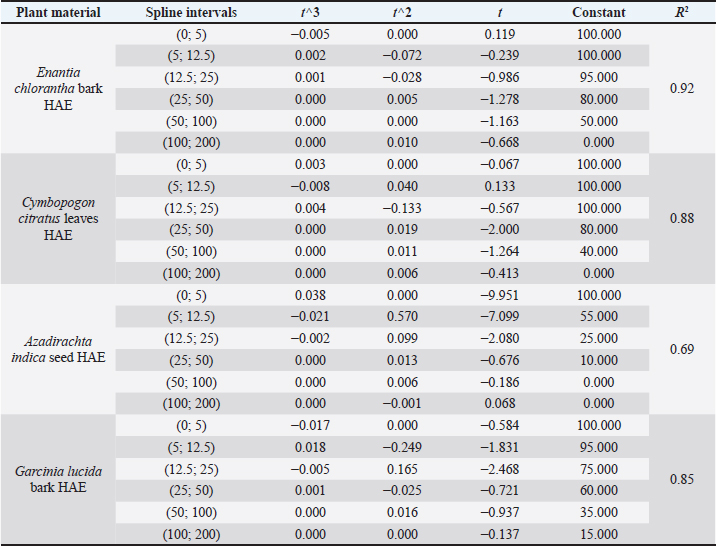
Fig. 1. (A): Group of 20 larvae. (B): Injection of GM larvae with plant extracts through the last left proleg. Table 1. Extract yields (%) obtained from the seven plants with distilled water and ethanolic solution (EtOH 80%).
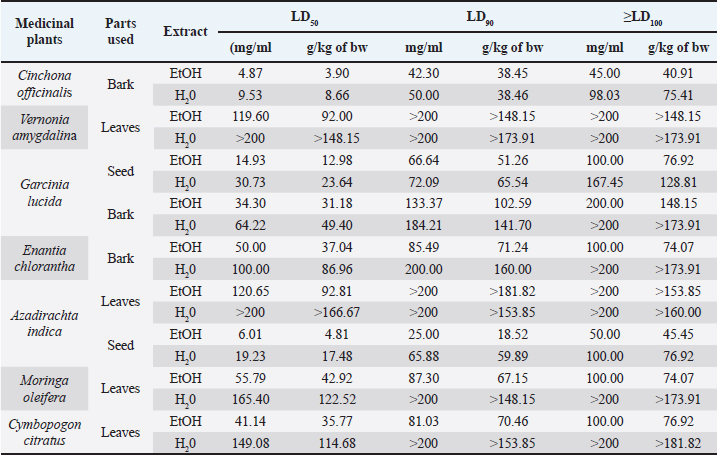
It is important to remember that the LD values were obtained using the greater wax moth (GM) as a toxicity assessment model. After 24 hours of incubation, survival data for each concentration of each plant material was used to plot spline cubic survival curves (examples of which are shown in Fig. 2) and to obtain the spline cubic equations (examples of which are presented in Table 2). As expected, no deaths were observed in larvae injected with 20 μl of PBS. However, as shown in Table 3, the LD50 (mg/ml) of the extracts tested varied from 4.87 [90 g/kg body weight (bw)] to >200 (> 166.67 g/kg bw), the LD90 (mg/ml) from 25.00 (18.52 g/kg bw) to >200 (> 181.82 g/kg bw) and LD100 (mg/ml) from 45.00 (40.91 g/kg bw) to > 200 (>181.82 g/kg bw). This difference between the LD values confirmed the good sensitivity of GM, which was reported as changing depending on the substances tested (Megaw et al., 2015; Ignasiak and Maxwell, 2017). The extracts with the highest toxicity were C. officinalis ethanolic bark extract (LD50=3.90 g/kg bw) followed by A. indica ethanolic seed extract (LD50=4.81 g/kg bw) and C. officinalis aquous bark extract (LD50=8.66 g/kg bw). However, other plant material such as water extract of leaves of C. citratus, M. oleifera, and V. amygdalina exerted the lowest toxicity on the greater wax moth with LD50 (mg/ml) of 149.08; 165.40 and > 200 respectively. The high toxicity of both ethanolic and aqueous extract of C. officinalis bark can be attributed to its composition having abundant quinine and its derivatives (dihydroquinine, cinchonidine, epiquinin, quinidine, dihydroquinidine, cinchonine, and epiquinidine) (Júnior et al., 2012; Bharadwaj et al., 2018) which are slightly soluble in water and soluble in ethanol (Gaponenko et al., 2020). Indeed, Quinine, C20H24N2O2, (8S, 9R) -6′-methoxycinchonana-9ol;(αR)-α-(6-methoxy-4-quinoyl)-α-[(2S, 4S, 5R)-(5-vinylquinuclidin-20yl)] methanol, is the most important alkaloid occurring in Cinchona (Maurice, 2014). We did not find any study in the literature evaluating the toxicity of extracts of C. officinalis but quinine was reported to have an LD50 of 1.392, 0.660 and 0.641 g/kg bw when administered orally to rats, mice, and rabbits, respectively (ECHA, 2021). These values differed to ours probably because the results reported by ECHA (2021) were those of pure quinine while our study focused on extracts with heterogeneous composition. Furthermore, and interestingly, we observed that some larvae exposed to concentrations greater than the LD100 of ethanolic extracts of C. officinalis bark exhibited localized melanization, whereas larvae injected by lower concentrations had slight and uniform melanization (Fig. 3). These results are similar to those obtained by Megaw et al. (2015) who used the GM model to test the toxicity of 1-alkyl-3-methylimidazolium chloride ionic liquids. Megaw et al. (2015) reported that melanization is a common component of the insect immune response, which occurs as a result of stress or infection, and leads to the larvae changing from cream-colored to dark brown or black. The two types of melanization in our study (Fig. 3B and C) can be explained by the fact that the high concentrations of the extracts were poorly distributed within the larvae unlike the low concentrations. Table 2. Cubic spline model used to determine the LD50, LD90 and LD100 of E. chlorantha bark ethanolic extract (HAE), C. citratus leaves HAE, A. indica seed HAE and G. lucida bark HAE.
In addition, similarly to our study, seeds of A. indica have been reported to have high toxicity both in animal models (Braga et al., 2021) and in insects (Islas et al., 2020) due to phytochemicals that they contain, including azadirachtin, which is used in the composition of certain natural insecticides (Tofel et al., 2017). This substance acts mainly by intercepting metamorphosis from the larval to adult stage and paralyzes the digestive tract of the larva (Tofel et al., 2017; Meisyara et al., 2019). Azadirachtin has been reported to be slightly soluble in water and freely soluble in polar organic solvents such as methanol, ethanol and ethyl acetate (Kale et al., 2020), which could explain the large difference between the LD50s of the. aqueous extract and the ethanolic extract of A. indica seed which were respectively 19.01 and 6.01 mg/ml. However, both ethanolic and aqueous extracts from the leaves of A. indica have demonstrated more than 10 times less toxicity than the seeds, possibly due to their different phytoconstituent composition. According to the classification of Gosselin, Smith and Hodge (CCOHS, 2021), biologically active compounds can be super toxic (LD50 <5 mg/kg bw), extremely toxic [LD50 ϵ (5–50 mg/kg bw)], very toxic [LD50 ϵ (50–500 mg/kg bw)], moderately toxic [LD50 ϵ (0.5–5 g/kg bw)], slightly toxic [LD50 ϵ (5–15 g/kg)], and practically non-toxic (above 15 g/kg). Therefore, the data from our study indicate that ethanolic extract of C. officinalis bark and A. indica seed are both moderately toxic, C. officinalis aqueous bark extract and G. lucida ethanolic seed extract are slightly toxic while all the other plant materials extracts are practically non-toxic. Notwithstanding that the results of this study corroborate with some of those previously reported (for the same plants on vertebrate models), further investigations are required under the same experimental conditions (in terms of preparation of the extracts) to determine the correlation between the LD50s in animal models and in GM. Finally, given the large number of larvae used in this study (1,100 larvae), this model may be of great utility if it is standardized as a study model for toxicity and may avoid animal sacrifices.
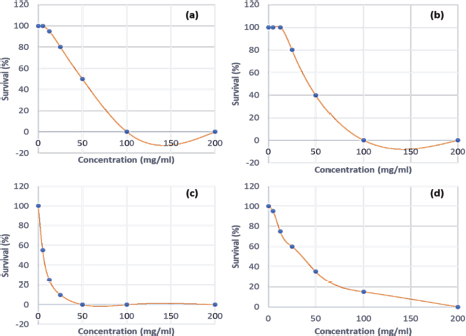
Fig. 2. Survival curves for GM larvae against (a): Enantia chlorantha ethanolic bark extract (HAE), (b): Cymbopogon citratus leaves HAE, (c): Azadirachta indica seed HAE (d): Garcinia lucida bark HAE. Each data point represents the mean percentage survival of 3 groups of 20 larvae, following injection with 20 μl of specific concentrations of the selected plant extract and incubation for 24 hours at 37°C. Table 3. LD50, LD90 and LD100 values for the medicinal plants tested on GM model.
Fig. 3. (A): Healthy GM larva. (B): Larva showing slight and uniform melanisation. (C): Larvae showing localised melanization following injection with a LD100 of C. officinalis ethanolic bark extract. ConclusionThe data presented in this study indicate that the aqueous and ethanolic extracts of barks from C. officinalis as well as the ethanolic extract of seeds from A. indica and G. lucida induced a high toxic effect on GM larvae; while other plant material such as water extract of M. oleifera, C. citratus, and V. amygdalina leaves exerted low toxicity. The high variability of the LD values from one plant to another demonstrates the good sensitivity of GM and allows us to conclude that GM can be used as a reliable system model for the assessment of the toxicity of medicinal plants. However, further studies are needed to establish the exact correlation between LD50, LD90, LD100 values in G. melonella and in vertebrate models in order to facilitate reliable and precise extrapolations in humans. AcknowledgmentsThis study has been supported by the RUDN University Strategic Academic Leadership Program. Conflict of interestThe authors declare that they have no competing interests. ReferencesAbbas, S., Nasreen, S., Haroon, A. and Ashraf, M.A. 2020. Synhesis of silver and copper nanoparticles from plants and application as adsorbents for naphthalene decontamination. Saudi. J. Biol. Sci. 27(4), 1016–1023. Abike, T.O., Osuntokun, O.T., Modupe, A.O., Adenike, A.F. and Atinuke, A.R. 2020. Antimicrobial efficacy, secondary metabolite constituents, ligand docking of Enantia chlorantha on selected multidrug resistance bacteria and fungi. J. Adv. Biol. Biotechnol. 23(6), 17–32. Aderinola, T.A., Alashi, A.M., Nwachukwu, I.D., Fagbemi, T.N., Enujiugha, V.N. and Aluko, R.E. 2020. In vitro digestibility, structural and functional properties of Moringa oleifera seed proteins. Food. Hydrocoll. 101, 105574. Alara, O.R. and Abdurahman, N.H. 2021. Vernonia amygdalina leaf and antioxidant potential. In Toxicology, Eds.,Vinood, B.P. and Victor, R.P. Cambridge, MA: Academic Press, pp: 347–353. Allegra, E., Titball, R.W., Carter, J. and Champion, O.L. 2018. Galleria mellonella larvae allow the discrimination of toxic and non-toxic chemicals. Chem. 198, 469–472. Anwar, F., Latif, S., Ashraf, M. and Gilani, A.H. 2007. Moringa oleifera: a food plant with multiple medicinal uses. Phytother. Res. 21(1), 17–25. Arévalo-Híjar, L., Aguilar-Luis, M.Á., Caballero-García, S., Gonzáles-Soto, N. and Valle-Mendoza, D. 2018. Antibacterial and cytotoxic effects of Moringa oleifera (Moringa) and Azadirachta indica (Neem) methanolic extracts against strains of Enterococcus faecalis. Int. J. Dent. 25, 1071676. Arsene, M.M.J., Viktorovna, P.I., Davares, A.K.L., Esther, N. and Nikolaevich, S.A. 2021. Urinary tract infections: virulence factors, resistance to antibiotics, and management of uropathogenic bacteria with medicinal plants—a review. J. Appl. Pharm. Sci. 11(7), 1–12. Arulselvan, P., Tan, W.S., Gothai, S., Muniandy, K., Fakurazi, S., Esa, N.M., Alarfaj, A.A. and Kumar, S.S. 2016. Anti-inflammatory potential of ethyl acetate fraction of Moringa oleifera in downregulating the NF-κB signaling pathway in lipopolysaccharide-stimulated macrophages. Molecules. 21(11), 1452. Baildya, N., Khan, A.A., Ghosh, N.N., Dutta, T. and Chattopadhyay, A.P. 2021. Screening of potential drug from Azadirachta Indica (Neem) extracts for SARS-CoV-2: an insight from molecular docking and MD-simulation studies. J. Mol. Struct. 1227, 129390. Bharadwaj, K.C., Gupta, T. and Singh, R.M. 2018. Alkaloid group of Cinchona officinalis: structural, synthetic, and medicinal aspects. In Synthesis of medicinal agents from plants, Eds., Ashish, T. and Supriya, T. Amsterdam, Netherlands: Elsevier, pp: 205–227. Braga, T.M., Rocha, L., Chung, T.Y., Oliveira, R.F., Pinho, C., Oliveira, A.I., Morgado, J. and Cruz, A. 2021. Azadirachta indica A. Juss. In vivo toxicity—an updated review. Molecules 26(2), 252. CCOHS (Canadian Centre for Occupational Health and Safety Act). 2021. What is a LD50 and LC50? CCOHS. Retrieved June 02, 2021. Available via https://www.ccohs.ca/oshanswers/chemicals/ld50.html Chinnasamy, G., Chandrasekharan, S., Koh, T.W. and Bhatnagar, S. 2021. Synthesis, characterization, antibacterial and wound healing efficacy of silver nanoparticles from Azadirachta indica. Front. Microbiol. 12, 204. Do, D.N., Nguyen, D.P., Phung, V.D., Le, X.T., Le, T.M., Do, V.M., Minh, B.Q. and Luu, X.C. 2021. Fractionating of lemongrass (Cymbopogon citratus) essential oil by vacuum fractional distillation. Processes 9(4), 593. Dumas, N.G.E., Anderson, N.T.Y., Godswill, N.N., Thiruvengadam, M., Ana-Maria, G., Ramona, P., Crisan, G.C., Laurian, V., Shariati, M.A., Tokhtarov, Z. and Emmanuel, Y. 2021. Secondary metabolite contents and antimicrobial activity of leaf extracts reveal genetic variability of Vernonia amygdalina and Vernonia calvoana morphotypes. Biotechnol. Appl. Biochem. 68(4), 938–947. ECHA (European Chemicals Agency). 2021. ECHA. Retrieved June 1, 2021. Available via https://echa.europa.eu/registration-dossier/-/registered-dossier/10428/7/3/1 Egbuonu, A.C.C. and Amadi, R.P. 2021. Ethanolic extract of ground Vernonia amygdalina stem exhibited potent antibacterial activity and improved hematological bio-functional parameters in normal and monosodium glutamate-intoxicated Rats. J. Appl. Sci. Environ. Manag. 25(3), 311–317. Etame, R.M.E., Mouokeu, R.S., Poundeu, F.S.M., Voukeng, I.K., Cidjeu, C.L.P., Tiabou, A.T., Yaya, A.J.G., Ngane, R.A.N., Kuiate, J.R. and Etoa, F.X. 2019. Effect of fractioning on antibacterial activity of n-butanol fraction from Enantia chlorantha stem bark methanol extract. BMC. Complement. Altern. Med. 19(1), 1–7. Gaponenko, Y., Mialdun, A. and Shevtsova, V. 2020. Diffusion of quinine with ethanol as a co-solvent in supercritical CO2. Molecules 25(22), 5372. Guedje, N.M. and Fankap, R. 2001. Utilisations traditionnelles de Garcinia lucida et Garcinia kola (Clusiaceae) au Cameroun. In Systematics and geography of plants, pp: 747–758. Guedje, N.M., Tadjouteu, F., Onana, J.M., Nga, E.N. and Ndoye, O. 2017. Garcinia lucida Vesque (Clusiaceae): from traditional uses to pharmacopeic monograph for an emerging local plant-based drug development. J. Appl. Biosci. 109, 10594–10608. Hanaa, A.M., Sallam, Y.I., El-Leithy, A.S. and Aly, S.E. 2012. Lemongrass (Cymbopogon citratus) essential oil as affected by drying methods. Ann. Agric. Sci. 57(2), 113–116. Hasibuan, P.A.Z., Harahap, U., Sitorus, P. and Satria, D. 2020. The anticancer activities of Vernonia amygdalina Delile. Leaves on 4T1 breast cancer cells through phosphoinositide 3-kinase (PI3K) pathway. Heliyon 6(7), e04449. Ignasiak, K. and Maxwell, A. 2017. Galleria mellonella (greater wax moth) larvae as a model for antibiotic susceptibility testing and acute toxicity trials. BMC. Res. Notes.10(1), 1–8. Islas, J.F., Acosta, E., Zuca, G., Delgado-Gallegos, J.L., Moreno-Treviño, M.G., Escalante, B. and Moreno-Cuevas, J.E. 2020. An overview of Neem (Azadirachta indica) and its potential impact on health. J. Funct. Foods. 74, 104171. Joseph, J., Lim, V., Rahman, H.S., Othman, H.H. and Samad, N.A. 2020. Anti-cancer effects of Vernonia amygdalina: a systematic review. Trop. J. Pharm. Res. 19(8), 1775–1784. Júnior, W.S.F., Cruz, M.P., Dos-Santos, L.L. and Medeiros, M.F.T. 2012. Use and importance of quina (Cinchona spp.) and ipeca (Carapichea ipecacuanha (Brot.) L. Andersson): plants for medicinal use from the 16th century to the present. J. Herb. Med. 2(4), 103–112. Kale, M., Dhanokar, S., Aher, A., Gawali, S. and Dhikale, R. 2020. Azadirachtin: natures gift to mankind. Curr. Trends. Pharm. Pharm. Chem. 2(4), 40–44 Koné, C. 2020. Plantes médicinales dans la prise en charge des infections urinaires. Doctoral dissertation, Bamako, Mali, USTTB. Kuete, V., Dongfack, M.D., Mbaveng, A.T., L allemand, M.C., Van-Dufat, H.T., Wansi, J.D., Seguin, E., Tillequin, F. and Wandji, J. 2010. Antimicrobial activity of the methanolic extract and compounds from the stem bark of Drypetes tessmanniana. Chin. J. Integr. Med. 16(4), 337–343. Land, M.H., Toth, M.L., MacNair, L., Vanapalli, S.A., Lefever, T.W., Peters, E.N. and Bonn-Miller, M.O. 2020. Effect of cannabidiol on the long-term toxicity and lifespan in the preclinical model Caenorhabditis elegans. Cannabis. Cannabinoid. Res. ahead of print. http://doi.org/10.1089/can.2020.0103. Maurice, M.I. 2014. Pharmacognostical profile of selected medicinal plants. In Handbook of African medicinal plants. Eds., Homas, B., Patricia, R. and John, D.L.P. Boca Raton, FL: CRC Press, Routledge Handbooks Online. Retrieved June 1, 2021. Available via https://www.routledgehandbooks.com/doi/10.1201/b16292-4 Mbarga, M.J.A., Podoprigora, I.V., Davares, A.K., Razan, M., Das, M.S. and Senyagin, A.N. 2021. Antibacterial activity of grapefruit peel extracts and green-synthesized silver nanoparticles. Vet. World. 14(5), 1330–1341. Megaw, J., Thompson, T.P., Lafferty, R.A. and Gilmore, B.F. 2015. Galleria mellonella as a novel in vivo model for assessment of the toxicity of 1-alkyl-3-methylimidazolium chloride ionic liquids. Chem. 139, 197–201. Meisyara, D., Krishanti, N.P.R.A., Zulfitri, A., Lestari, A.S., Tarmadi, D., Himmi, S.K., Amin1, Y., Zulfiana1, D., Fajar1, A., Yusuf, S. and Ismayati, M. 2019. Biological activity of local plant extracts from Toba Region as insecticide. IOP Conf. Series. Earth. Environ. Sci. 374(1), 012006. Mouafo, H.T., Tchuenchieu, A.D.K., Nguedjo, M.W., Edoun, F.L.E., Tchuente, B.R.T. and Medoua, G.N. 2021. In vitro antimicrobial activity of Millettia laurentii De Wild and Lophira alata Banks ex CF Gaertn on selected foodborne pathogens associated to gastroenteritis. Heliyon 7(4), e06830. Moya-Andérico, L., Vukomanovic, M., del Mar Cendra, M., Segura-Feliu, M., Gil, V., José, A. and Torrents, E. 2021. Utility of Galleria mellonella larvae for evaluating nanoparticle toxicology. Chem. 266, 129235. Muala, W.C.B., Desobgo, Z.S.C. and Jong, N.E. 2021. Optimization of extraction conditions of phenolic compounds from Cymbopogon citratus and evaluation of phenolics and aroma profiles of extract. Heliyon 7(4), e06744. Nagar, N. and Devra, V. 2018. Green synthesis and characterization of copper nanoparticles using Azadirachta indica leaves. Mater. Chem. Phys. 213, 44–51. Ogungbemi, A.O. and van Gestel, C.A. 2018. Extrapolation of imidacloprid toxicity between soils by exposing Folsomia candida in soil pore water. Ecotoxicol. 27(8), 1107–1115. Ohemu, T.L., Agunu, A., Chollom, S.C., Okwori, V.A., Dalen, D.G. and Olotu, P.N. 2018. Preliminary phytochemical screening and antiviral potential of methanol stem bark extract of Enantia chlorantha Oliver (Annonaceae) and Boswellia dalzielii Hutch (Burseraceae) against Newcastle disease in Ovo. Eur. J. Med. Plants. 25(4), 1–8. Olivier, D.K., Van Vuuren, S.F. and Moteetee, A.N. 2015. Annickia affinis and A. chlorantha (Enantia chlorantha)–a review of two closely related medicinal plants from tropical Africa. J. Ethnopharmacol. 176, 438–462. Piatek, M., Sheehan, G. and Kavanagh, K. 2020. Utilising Galleria mellonella larvae for studying in vivo activity of conventional and novel antimicrobial agents. Pathog. Dis. 78(8), ftaa059; doi:10.1093/femspd/ftaa059 Ray, S.J., Wolf, T.J. and Mowa, C.N. 2015. Moringa oleifera and inflammation: a mini-review of its effects and mechanisms. Acta Hortic. 1158, 317-330. Saleem, A., Saleem, M. and Akhtar, M.F. 2020. Antioxidant, anti-inflammatory and antiarthritic potential of Moringa oleifera Lam: an ethnomedicinal plant of Moringaceae family. S. Afr. J. Bot. 128, 246–256. Situmorang, P.C., Ilyas, S., Hutahaean, S. and Rosidah, R. 2020. Components and acute toxicity of nanoherbal haramonting (Rhodomyrtus tomentosa). J. Herb. Med. Pharmacol. 10(1), 139–148. Sonfack, C.S., Nguelefack-Mbuyo, E.P., Kojom, J.J., Lappa, E.L., Peyembouo, F.P., Fofié, C.K., Nolé, T., Nguelefack, T.B. and Dongmo, A.B. 2021. The aqueous extract from the stem bark of Garcinia lucida Vesque (Clusiaceae) exhibits cardioprotective and nephroprotective effects in adenine-induced chronic kidney disease in rats. Evid. Based. Complement. Alternat. Med. 2021, 5581041. Sylvie, D.D., Anatole, P.C., Cabral, B.P. and Veronique, P.B. 2014. Comparison of in vitro antioxidant properties of extracts from three plants used for medical purpose in Cameroon: Acalypha racemosa, Garcinia lucida and Hymenocardia lyrata. Asian. Pac. J. Trop. Biomed. 4, S625–S632. Tofel, K.H., Kosma, P., Stähler, M., Adler, C. and Nukenine, E.N. 2017. Insecticidal products from Azadirachta indica and Plectranthus glandulosus growing in Cameroon for the protection of stored cowpea and maize against their major insect pests. Ind. Crops. Prod. 110, 58–64. Tonukari, N.J., Avwioroko, O.J., Ezedom, T. and Anigboro, A.A. 2015. Effect of preservation on two different varieties of Vernonia amygdalina Del.(bitter) leaves. Food. Sci. Nutr. 6(7), 623. Vijayakumar, S., Divya, M., Vaseeharan, B., Ranjan, S., Kalaiselvi, V., Dasgupta, N., Chen, J. and Durán-Lara, E.F. 2020. Biogenic preparation and characterization of ZnO nanoparticles from natural polysaccharide Azadirachta indica. L. (neem gum) and its clinical implications. J. Clust. Sci. 32, 983–993. Wang, W.T., Liao, S.F., Wu, Z.L., Chang, C.W. and Wu, J.Y. 2020. Simultaneous study of antioxidant activity, DNA protection and anti-inflammatory effect of Vernonia amygdalina leaves extracts. PLoS. One. 15(7), e0235717. Wijesinghe, G.K., Maia, F.C., de Oliveira, T.R., de Feiria, S.N.B., Joia, F., Barbosa, J.P., Boni, G.C., Sardi, J.C.O., Rosalen, P.L. and Höfling, J.F. 2020. Effect of Cinnamomum verum leaf essential oil on virulence factors of Candida species and determination of the in-vivo toxicity with Galleria mellonella model. Mem. Inst. Oswaldo. Cruz. 115, e200349. Yedjou, C.G., Sims, J.N., Njiki, S., Tsabang, N., Ogungbe, I.V. and Tchounwou, P.B. 2018. Vernonia amygdalina delile exhibits a potential for the treatment of acute promyelocytic leukemia. Glob. J. Adv. Eng. Technol. Sci. 5(8), 1–9. Zhang, Q., Hao, L. and Hong, Y. 2021. Exploring the multilevel effects of triclosan from development, reproduction to behavior using Drosophila melanogaster. Sci. Total. Environ. 762, 144170. | ||
| How to Cite this Article |
| Pubmed Style Arsene MMJ, Viktorovna PI, Davares AKL. Galleria mellonella (greater wax moth) as an eco-friendly in vivo approach for the assessment of the acute toxicity of medicinal plants: application to some plants from Cameroon. Open Vet. J.. 2021; 11(4): 651-661. doi:10.5455/OVJ.2021.v11.i4.15 Web Style Arsene MMJ, Viktorovna PI, Davares AKL. Galleria mellonella (greater wax moth) as an eco-friendly in vivo approach for the assessment of the acute toxicity of medicinal plants: application to some plants from Cameroon. https://www.openveterinaryjournal.com/?mno=100562 [Access: January 25, 2026]. doi:10.5455/OVJ.2021.v11.i4.15 AMA (American Medical Association) Style Arsene MMJ, Viktorovna PI, Davares AKL. Galleria mellonella (greater wax moth) as an eco-friendly in vivo approach for the assessment of the acute toxicity of medicinal plants: application to some plants from Cameroon. Open Vet. J.. 2021; 11(4): 651-661. doi:10.5455/OVJ.2021.v11.i4.15 Vancouver/ICMJE Style Arsene MMJ, Viktorovna PI, Davares AKL. Galleria mellonella (greater wax moth) as an eco-friendly in vivo approach for the assessment of the acute toxicity of medicinal plants: application to some plants from Cameroon. Open Vet. J.. (2021), [cited January 25, 2026]; 11(4): 651-661. doi:10.5455/OVJ.2021.v11.i4.15 Harvard Style Arsene, M. M. J., Viktorovna, . P. I. & Davares, . A. K. L. (2021) Galleria mellonella (greater wax moth) as an eco-friendly in vivo approach for the assessment of the acute toxicity of medicinal plants: application to some plants from Cameroon. Open Vet. J., 11 (4), 651-661. doi:10.5455/OVJ.2021.v11.i4.15 Turabian Style Arsene, Mbarga Manga Joseph, Podoprigora Irina Viktorovna, and Anyutoulou Kitio Linda Davares. 2021. Galleria mellonella (greater wax moth) as an eco-friendly in vivo approach for the assessment of the acute toxicity of medicinal plants: application to some plants from Cameroon. Open Veterinary Journal, 11 (4), 651-661. doi:10.5455/OVJ.2021.v11.i4.15 Chicago Style Arsene, Mbarga Manga Joseph, Podoprigora Irina Viktorovna, and Anyutoulou Kitio Linda Davares. "Galleria mellonella (greater wax moth) as an eco-friendly in vivo approach for the assessment of the acute toxicity of medicinal plants: application to some plants from Cameroon." Open Veterinary Journal 11 (2021), 651-661. doi:10.5455/OVJ.2021.v11.i4.15 MLA (The Modern Language Association) Style Arsene, Mbarga Manga Joseph, Podoprigora Irina Viktorovna, and Anyutoulou Kitio Linda Davares. "Galleria mellonella (greater wax moth) as an eco-friendly in vivo approach for the assessment of the acute toxicity of medicinal plants: application to some plants from Cameroon." Open Veterinary Journal 11.4 (2021), 651-661. Print. doi:10.5455/OVJ.2021.v11.i4.15 APA (American Psychological Association) Style Arsene, M. M. J., Viktorovna, . P. I. & Davares, . A. K. L. (2021) Galleria mellonella (greater wax moth) as an eco-friendly in vivo approach for the assessment of the acute toxicity of medicinal plants: application to some plants from Cameroon. Open Veterinary Journal, 11 (4), 651-661. doi:10.5455/OVJ.2021.v11.i4.15 |