| Original Article | ||
Open Vet. J.. 2021; 11(4): 734-746 Open Veterinary Journal, (2021), Vol. 11(4): 734–746 Original Research Hematological and biochemical profiles of canine CD45− T lymphomas are different from other immunophenotypesRosina Sánchez-Solé1*, Florencia Mosquillo2, Paulo Jark3, Martín Breijo4 and Paula Pessina11Laboratorio de Análisis Clínicos, Departamento de Clínicas y Hospital Veterinario, Facultad de Veterinaria, Universidad de la República, Montevideo, Uruguay 2Laboratorio de Interacciones Moleculares, Instituto de Química Biológica e Instituto de Biología, Facultad de Ciencias, Universidad de la República, Montevideo, Uruguay 3Onconnectionvet, Nova Aliança, Ribeirão Preto, São Paulo, Brazil 4Unidad de Reactivos y Biomodelos de Experimentación, Facultad de Medicina, Universidad de la República, Montevideo, Uruguay *Corresponding Author: Rosina Sánchez-Solé. Laboratorio de Análisis Clínicos, Departamento de Clínicas y Hospital Veterinario, Facultad de Veterinaria, Universidad de la República, Montevideo, Uruguay. Email: rosinasanchsole [at] gmail.com Submitted: 07/09/2021 Accepted: 23/11/2021 Published: 21/12/2021 © 2021 Open Veterinary Journal
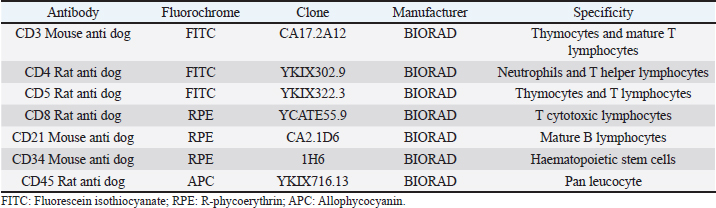
AbstractBackground: Canine multicentric lymphomas are lymphoproliferative malignancies that have increased in recent decades. The patient’s treatment and prognosis are determined by the grade, histological type, and lymphoma immunophenotyping. Aim: To investigate the paraclinical signs and survival time in canines with different lymphoma immunophenotypes. Methods: Over 2 and a half years, 47 untreated dogs were diagnosed with multicentric lymphoma at the Veterinary School Hospital of Uruguay. The disease was clinically and cytologically diagnosed, and immunophenotyping was determined by flow cytometry. After the immunophenotyping, most of the patients were grouped into the following: B (LB), T aggressive (LTCD45+), or T-zone lymphoma (LTCD45−). The patients’ haematological values, calcemia, lactate dehydrogenase (LDH) levels, and plasmatic electrophoretic profiles were all determined immediately after that. Results: Of all canine lymphomas, 55.3% were B, 31.9% were LTCD45+, and 10.6% were TCD45−. Only 2.2% were classified as nonB/nonT, and survival time differed between groups. Patients with LTCD45− lymphomas had a mean life span of 641 days after diagnosis, followed by LB (166 days) and LTCD45+ (62 days). Red blood cell count, hematocrit, and hemoglobin levels did not differ between groups. However, the LTCD45− group had significantly higher lymphocyte levels than the LTCD45+ and LB groups (p=0.01 and 0.006, respectively). Levels of albumin, alpha-1, and alpha-2 globulins did not differ between groups. On the other hand, gamma globulins levels in the LTCD45− were higher than in the other lymphoma groups. The presence of hypercalcemia and high plasma LDH levels were associated with patient severity. Only the TCD45+ group had hypercalcemia although both the LB and TCD45+ groups had elevations in LDH activity. Interestingly, there was a direct relationship between high LDH values (greater than 500 IU/l) and lower survival in TCD45+ lymphomas. Conclusion: Survival time and hematological and biochemical patterns differed among canine lymphomas immunophenotypes. Patients of LTCD45− phenotype showed higher lymphocyte counts and gamma globulin levels and more prolonged survival. Serum LDH activity may provide additional prognostic information in high-grade T-cell lymphoma. Keywords: Dogs, Immunophenotype, Multicentric lymphoma, Paraneoplastic syndromes, Survival times. IntroductionThe incidence of malignant lymphomas, both in humans and canines, has increased in the last decades. In dogs, they represent between 7% and 24% of all neoplasms (Vail et al., 2013) and 80% of hematopoietic tumors (Ponce et al., 2010; Aniolek et al., 2014). It most often affects middle-aged or elderly dogs (Dorn et al., 1967; Merlo et al., 2008), and the most predisposed breeds are Doberman, Rottweiler, Boxer, and Bernese Mountain Dog (Comazzi et al., 2018). Lymphoma has multifactorial causes, but genetic or environmental factors increase its incidence (Breen and Modiano, 2008; Marconato et al., 2009; Pastor et al., 2009). Patient survival depends on the type of lymphoma and the degree of disease progression. Most canine lymphomas arise from B-cells and are usually characterized by the expression of CD21 and CD79a (Ruslander et al., 1997; Valli et al., 2011; Ernst et al., 2016; Pawlak et al., 2016). A smaller proportion are T-cell lymphomas, identified by the expression of CD3 and CD5 markers and, depending on the T-cell subtypes, by CD4 or CD8 markers (Avery et al., 2014). In addition, there is a small portion of lymphomas that do not express labeling for T or B cells, identified as null cell lymphoma (Ponce et al., 2010). In general, high-grade T-cell lymphoma has a worse prognosis than high-grade B-cell lymphomas (Kiupel et al., 1999; Beaver et al., 2010; Papakonstantinou et al., 2013; Aresu et al., 2015). However, low-grade T-cell lymphoma, overrepresented by T-zone lymphoma (TZL), shows a better prognosis and a less aggressive clinical course. This variation of T-cell lymphomas represents about 10%–15% of the T-cell phenotype (Ponce et al., 2004; Valli et al., 2006; Avery et al., 2014; Jark et al., 2020). Canine small clear cell / TZLs are associated with the absence of the CD45 marker (Seelig et al., 2014). These are defined histopathologically by small or intermediate cells with low mitotic index. Lymphocytes have a “hand mirror form” that enlarges the paracortex and medullary cords while leaving the nodal architecture intact (Ponce et al., 2004; Valli et al., 2011). CD45− T-cell lymphoma generally showed aberrant immunophenotypes, with CD4−CD8− being the most frequent (Pawlak et al., 2016; Harris et al., 2020). There is little information on why CD45− patients have better survival. The CD45 marker is considered a hallmark in diagnosing the disease (Martini et al., 2015, 2017). Hematological and biochemical alterations have been observed in patients with lymphoma. Anemia and thrombocytopenia have been associated with poor prognosis (Abbo and Lucroy, 2007; Miller et al., 2009). Meanwhile, it was reported that canines with neutrophilia aggravate the evolution of the disease (Gavazza et al., 2008; Perry et al., 2011; Calvalido et al., 2016; Curran and Thamm, 2016). Mature lymphocytosis is a common finding in TZL (up to 50% of dogs) but does not affect prognosis (Seelig et al., 2014; Martini et al., 2016). As suggested, this could be due to the overflow of nodal lymphocytes into the bloodstream, as the loss of CD45 renders galectin unable to bind, evading apoptosis activation and prolonging lymphocyte survival (Hernandez and Baum, 2002; Seelig et al., 2014; Martini et al., 2016). Hypercalcemia and gammopathies are paraneoplastic syndromes frequently associated in dogs with lymphomas (Vail et al., 2013). Also, increased lactate dehydrogenase (LDH) activity suggests a poor prognosis and has been associated with the presence of relapses (Zanatta et al., 2003; Marconato et al., 2010). Although the paraclinical parameter provides us with non-specific information, it allows the clinician to assess the severity of the disease. The prognosis of canine lymphoma depends on many factors, but the immunophenotype of the lymphoma is one of the most important (Marconato et al., 2011; Valli et al., 2013). The median survival of canines with indolent T-cell (low-grade) lymphoma is up to three times that of high grade B- or T-cell lymphomas (Valli et al., 2013). Unfortunately, unlike in humans (non-Hodgkin lymphoma), there are no veterinary prognostic scoring systems routinely used for canine lymphoma (Fontaine et al., 2017). Despite this, some biochemical parameters have been studied with controversial results in search of additional prognostic value. This study aimed to determine whether the hematologic or biochemical changes observed in dogs with lymphoma are related to their immunophenotypes (B, TCD45+, and TCD45−) or to their survival time. Materials and MethodsClinical cases of canine lymphomaA total of 47 untreated dogs seen at the Veterinary School Hospital of Uruguay between April 2018 and September 2020 with a clinical diagnosis of multicentric lymphoma were included in this study. The lymphoma diagnosis was confirmed by cytologic evaluation of the enlarged lymph nodes, while flow cytometry performed lymphoma immunophenotyping. All dogs were at least clinical stage III, except for one dog with LTCD45+, stage II. Unfortunately, chest X-rays, abdominal ultrasound, and bone marrow aspirations were not available in all cases, which did not accurately determine stages IV and V lymphoma. The time from cytologic diagnosis to death or euthanasia was defined as their overall survival time (OST). Data collected from patients included breed, age, sex, reproductive status, genetic history, clinical examination findings, laboratory test results, cytologic examination, and imaging. Cytology and immunophenotypingCell samplesLymph node aspirates performed in each patient were used to confirm the cytologic diagnosis and to study the immunophenotype. Fine needle aspiration obtained punctures from the popliteal lymph nodes using a 5 ml disposable syringe and a 21-23G hypodermic needle. A clinical pathologist performed cytologic evaluation by May-Grunwald Giemsa stained smears. For immunophenotyping, lymph node aspirates were placed in phosphate-buffered saline and EDTA (2 nM, pH 7.4) at room temperature until use (no more than 2 hours) (Jalla et al., 2004). Sample staining and data collectionCell samples were counted, and the final volume was adjusted to 0.5–1×107 cells/ml. Propidium iodide (10 μg/ml–Invitrogen from Thermo Fisher Scientific) was used to determine cell viability, and only samples with vitality greater than 80% were included in the research. The lymphocytes were then incubated with 5 μl of each of the monoclonal antibodies conjugated to different fluorochromes in a dark place at room temperature for 45 minutes according to the manufacturer’s recommendations (BIORAD, CA). One ml of red cell lysing solution (QUICKLYSIS, Cytognos, Salamanca, Spain) was added to the samples and incubated for 10 minutes at room temperature. The panel of antibodies used to identify the cell line is shown in Table 1. All samples were analyzed in duplicate on a Becton Dickinson (BD) Accuri C6 flow cytometer (BD Biosciences, CA). An unstained tube was used as a negative control (Martini et al., 2020; Ferrari et al., 2021). For data collection, a lymphocyte gate was defined based on the complexity of the cytoplasm and cell dimension, depicted on the forward-scatter plot versus side scatter to allow good visualization of the different cell populations contained in the sample. A debris threshold was established to analyze the data, and a minimum of 5,000 cells was counted within the lymphocyte gate of each sample, as suggested by Lana et al. (2006). Parameter analysis was performed using a bandpass filter of 533/30 nm for FITC-conjugated antibodies (FL1), 585/40 nm for PE-conjugated antibodies (FL2), and 675/25 nm for the APC fluorophore (FL4). Single staining controls were used to calculate color compensation. The FL1 detector was corrected by subtracting a percentage of 4.75% from FL2, and the compensation value for the FL2 channel was 9.10%, deducted from FL1. The data were analyzed with CFlow Plus software (BD). Table 1. Monoclonal antibodies used for differentiation and labeling of canine lymphocytes by flow cytometry obtained from patients with multicentric lymphoma.
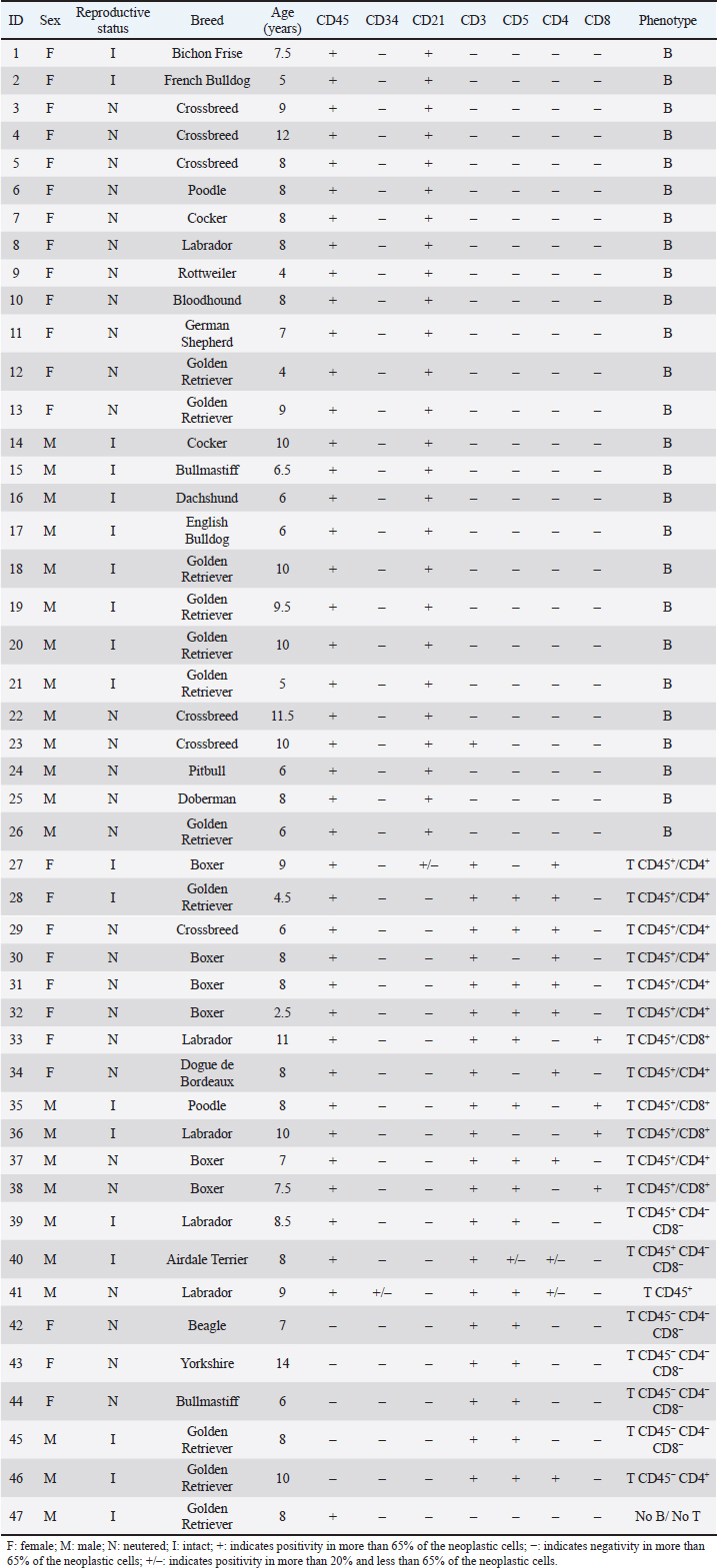
Hematological and biochemical determinationsBlood from dogs was collected by cephalic vein puncture. Samples were placed in tubes anticoagulated with EDTA (K3 plus ethylenediamine tetraacetic acid) and in dry tubes. Cellular blood variables [red blood cell (RBC), hemoglobin, hematocrit, white blood cell (WBC), neutrophils, lymphocytes, monocytes, eosinophils, platelet counts] were performed with a veterinary hematology analyzer (Mythic 18 Vet, Orphée, Geneva, Switzerland), and by microscopic evaluation of air-dried smears stained with May-Grunwald Giemsa. Biochemical analyses (calcemia, LDH activity, total proteins, and albumins) were performed on serum samples in a semiautomatic spectrophotometer (CB 350i-Wiener lab Group, Rosario, Argentina). To ensure that hypercalcemia was not due to lipemia or hemolysis, it was interpreted in relation to the serum albumin level, using a correction formula as suggested by Bergman (2012): adjusted calcium (mg/dl)=[Calcium (mg/dl)–albumin (g/dl)] + 3.5. The reference ranges used for these variables were from Oregon State University. Gammopathies were explored by capillary electrophoresis (Minicap, Sebia, Barcelona, Spain). The protein fractions were albumin and alpha 1, alpha 2, beta and gamma globulins. Ethical approvalThe Ethical Committee approved this investigation of the Faculty of Veterinary Medicine of Universidad de la República (University of the Republic) of Uruguay (CEUA FVET-801 111900-000780-19). In addition, the owners of the dogs gave their signed consent for the participation of their animals in this study. Statistical analysisAnalyses were performed with the statistical program PAST 4.03. Normal distribution was tested by the Shapiro–Wilk test. When the variables presented a normal distribution, the group effect (LB vs. LTCD45+ vs. LTCD45−) on hematological and biochemical variables was analyzed by one-way analysis of variance, and post hoc comparisons (differences between groups) were performed with Tukey’s test. When the variables did not have a normal distribution, a Kruskal–Wallis test was performed to evaluate the group effect. The Mann–Whitney test was used to compare groups. The proportions of the different hematological and biochemical abnormalities were analyzed according to the immunophenotype using Fisher’s exact tests. Kaplan–Meier curves were drawn and compared by the Long–Rank (Mantel–Cox) test to assess the influence of immunophenotype and LDH activity above and below 500 IU/l in LTCD45+ and LB phenotypes on overall survival. The prognostic value of LDH was not investigated in the LTCD45− subgroup because none of the dogs had LDH levels above 500 IU/l. Each step represents the death of individual cases. OST was calculated in days from the time of diagnosis by cytology to the time of death or censor (460 days) and expressed as hazard ratio (HR). The statistical software used was GraphPad Prism (GraphPad Software, 2365 Northside Dr., Suite 560, San Diego, CA 92108). Differences were considered significant when p ≤ 0.05. ResultsPatient population and immunophenotypingOf the 47 dogs with multicentric lymphoma, 22 were males (46.81%), and 25 were females (53.19%). The mean age of dogs with lymphoma was 7.87 years (range 2.5 to 14 years), and the most represented breeds were Golden Retriever (23.4%), Boxer, Labrador, and crossbreeds (12.8% each, respectively), (Table 2). Table 2. Population characteristics and immunophenotyping by flow cytometry of neoplastic populations identified in lymph node aspirates from 47 dogs with multicentric lymphoma.
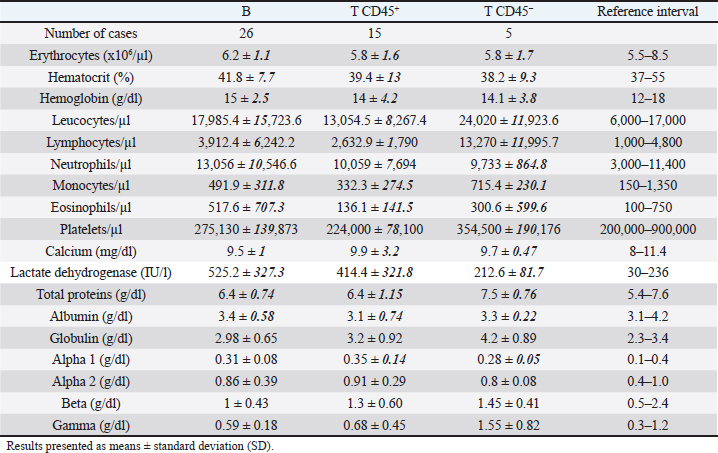
While 55.3% of the dogs were large B-cell lymphomas, 42.6% belonged to the T-cell immunophenotype. Within the T-cell lymphomas, 75% were CD45+ (15/20) and 25% were CD45− (indolent TZLs). Most of the CD45+ T-cell lymphomas were CD4+ (53.3%), the 26.7% were CD8+, and 13.3% CD4/CD8 double negative. One dog (6.7%) classified as CD45+ T (CD3+, CD5+) had weak CD4 expression (marking positivity in more than 20% and less than 65% of neoplastic cells) and was not included in any of the above subgroups. The CD45− T-cell group represented 10.64% of the total patients and consisted of small cells in the flow cytometry scatter plots. 80% (4/5) of the dogs were CD4/CD8 double negative, and the other 20% were CD4+. Interestingly, the mean age of this group at diagnosis was 9 years, higher than that of the B and T phenotypes (7.7 and 7.6 years, respectively). In this study, only one dog was classified as non-B and non-T-cell lymphoma, as it did not show positive marking to any of the specified lineages (Table 2). The chemotherapy treatment that each patient received after diagnosis depended on their treating clinician and the economic possibilities of their owners, with a wide variety of treatments for all phenotypes. Single-drug regimens (glucocorticoids, doxorubicin, lomustine, vincristine, and chlorambucil) as well as combos and multidrug protocols were used (Cyclophosphamide, vincristine, and prednisone or Cyclophosphamide, doxorubicin, vincristine, and prednisone). A total of 13 dogs did not receive treatment (LB, n=7; LTCD45+, n=3; LTCD45−, n=3). Table 3. Summary of blood cell counts and biochemical parameters in lymphomas of different phenotypes.
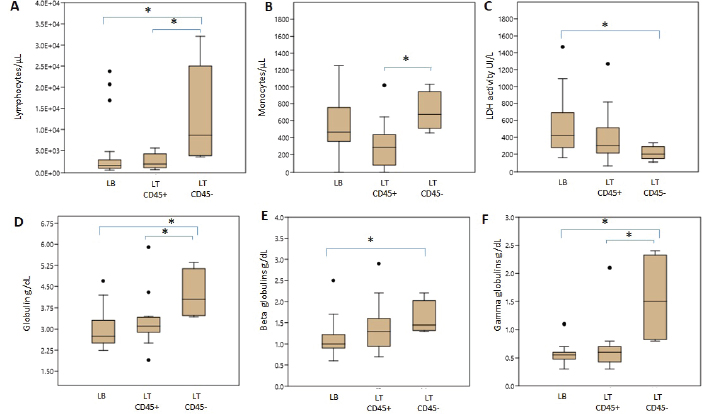
Hematologic variablesRBC counts, hematocrit, and hemoglobin concentration did not differ between canines with different lymphoma phenotypes (Table 3). In this study, 13 of 47 dogs with lymphoma had anemia. The distribution and degree of severity of anemia did not differ between groups. WBC counts were significantly different between T lymphoma subtypes. Dogs with LTCD45− lymphomas had higher leukocyte counts than with LTCD45+ (p=0.036). However, no significant differences in WBC counts were found between the other groups. Of the patients with lymphoma, 27.7% had leukocytosis, but the distribution between the groups was similar. Only two of the patients belonging to the LTCD45+ group had leukopenia. Lymphocyte counts did not differ between the LTCD45+ and LB groups. However, the LTCD45− group had significantly higher lymphocyte levels than the LTCD45+ and LB groups (p=0.01 and=0.006, respectively) (Fig. 1A). Canines with lymphocytosis were identified in 20% of cases. Sixty percent of the patients belonging to the LTCD45− group had mature lymphocytosis. On the other hand, 20% of the dogs had lymphopenia, and its distribution was similar between the groups. Mean eosinophil counts were higher in dogs with LB than those in the LTCD45+ group (p=0.049), but there was no difference with the LTCD45− group. There were also no differences between the LTCD45+ and LTCD45− groups. Eosinophilia was identified in 15.2% of patients. Eosinopenia was seen in 36.96% of patients with lymphoma, and the highest proportion was observed in the LTCD45− group (p=0.027). Mean monocyte counts were significantly different between groups (p=0.026). The LTCD45− group had more circulating monocytes than the LTCD45+ group (p=0.03) but did not differ from the LB group. There were also no differences between LB and LTCD45+ groups (Fig. 1B). Monocytopenia was shown in 21.74% of lymphoma patients and had a similar distribution between groups. There were no significant differences in neutrophil and platelet counts between either group. Neutrophilia and thrombocytopenia were observed in 26.1% and 32.5% of patients, respectively. No differences were found between the groups. Serum biochemistryCalcium levels were similar between groups. Hypercalcemia was observed in 4 of the 38 patients with lymphoma, and all of them belonged to the LTCD45+ group (p=0.018). Mean LDH activity differed significantly between groups (p=0.029), being significantly higher in LB than in LTCD45− (p=0.012), while there was no difference between the other groups (Fig. 1C). Increased LDH activity was found in 74.4% of patients. Moreover, 83.3% of LB, 69.2% of LTCD45+ and 40% of LTCD45− had increased LDH activity.
Fig. 1. Differences in blood cell counts and biochemical variables between LB, LT CD45+ and LT CD45− groups. A. Lymphocyte count. B. Monocyte count. C. LDH activity. D. Globulins. E. Beta globulins. F. Gamma globulins. Data are plotted as box-and-whisker plots and show outliers. Data are presented as means +/− SD. Asterisk indicates differences on the same graph, p < 0.05.
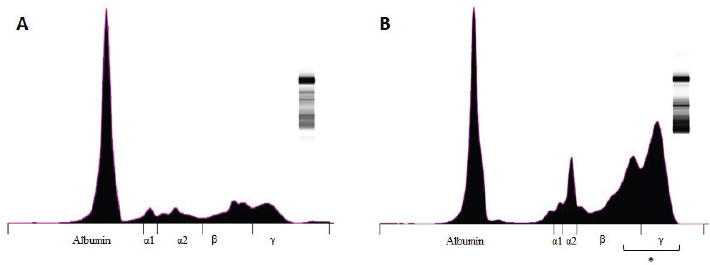
Fig. 2. Graphic representation of the capillary protein electrophoresis (A, LB; B, LTCD45−). The different protein fractions are observed (albumins; alpha-1 globulins, alpha-2 globulins; beta globulins, gamma globulins). A polyclonal gammopathy is distinguished, characterized by the presence of a broad-based beta-gamma peak (*). Plasma total protein concentrations were similar between groups. Also, when the protein fractions were studied, the mean levels of albumin, alpha-1, and alpha-2 globulins did not differ between groups (Table 3). However, LTCD45− had higher levels of gamma globulins than the LB and LTCD45+ groups (p=0.004 and 0.01, respectively), (Figs. 1D, F, and 2) and higher levels of beta globulins than the LB group (p=0.02), (Fig. 1E).
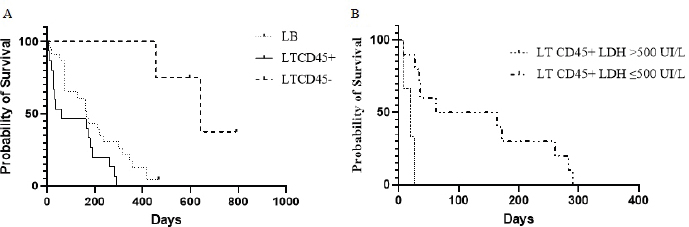
Fig. 3. Kaplan–Meier curves depicting overall lymphoma survival in 42 dogs divided into phenotypic groups (LB, n=23; LTCD45+, n=15; LTCD45−, n=4), (A); and in 13 dogs with LTCD45+ and different LDH activity (>500 IU/l, n=3; ≤500 IU/l, n=10), (B). “|”, live patients. SurvivalOST differed between groups ( p=0.0004). LTCD45− dogs survived longer than LTCD45+ and LB (p=0.001 and 0.0025, respectively), and in turn, LB dogs had a longer survival time than LTCD45+ (p=0.034) (Fig. 3A). The median survival time was 641 days (95% CI: 455–792) for LTCD45−, 166 days (95% CI: 14–418) for LB and 62 days (95% CI: 7–290) for LTCD45+. At the end of the study, one of the dogs in LB and two of the dogs in LTCD45− survived. The HR was 0.51 (95% CI: 0.25–1.07) between LB and LTCD45+, 5.72 (95% CI: 2.6–13.29) between LB and LTCD45−, and 6.45 (95% CI: 2.48–16.73) between LTCD45+ and LTCD45−. Decreased survival time was detected for LTCD45+ dogs with LDH activity above 500 IU/l compared to those with values below 500 IU/l (p=0.0018) (Fig. 3B). The median survival time was 113 days (95% CI: 69–437) for LDH values below 500 IU/l and 20 days (95% CI: 7–26) for values above 500 IU/l, and the HR was 5.44 (95% CI: 0.47–62.6). No significant differences were detected in survival time for LB dogs with LDH activity above or below 500 IU/l (p=0.30). DiscussionProper diagnosis of lymphomas allows veterinary clinicians to evaluate the best treatment and management of a disease that severely affects patient survival and well-being. In the present work, we explored the association between different immunophenotypes of lymphomas and paraclinical signs. This information could help us to understand or predict the development of the disease. Of the 47 dogs with multicentric lymphoma in the study, B-cell phenotype was diagnosed in 55.3% of the cases. In contrast, 42.5% of the lymphomas were of T-cell origin (10.64% TZL and 31.9% aggressive T lymphoma). Most of the previous works showed a high prevalence of phenotype B over T lymphomas (Teske et al., 1994; Ponce et al., 2010; Valli et al., 2011; Valli et al., 2013). However, the reported percentage of T-cell lymphomas in canines differs between regions. For example, European and North American studies showed the incidence of T-cell lymphomas to be approximately 30% (Fournel-Fleury et al., 1997; Ruslander et al., 1997; Calvalido et al., 2016; Pawlak et al., 2016). On the other hand, studies from several groups in Latin America found T immunophenotypes equal to or higher than those reported in the present study (Moreno and Bracarense, 2007; Álvarez-Berger et al., 2009; Neuwald et al., 2014). However, a recently published Brazilian paper reported frequencies similar to European and North American studies (Jark et al., 2020). The genetic background of dog populations and environmental factors could contribute to these regional differences (Modiano et al., 2005; Pastor et al., 2009; Ruple et al., 2017). Golden Retrievers and Boxers were the most common breeds in our research, which matches what has previously been reported in America (Modiano et al., 2005; Jark et al., 2020). Not surprisingly, Golden Retrievers were overrepresented in the LTCD45− group due to a notable predilection of the breed to develop TZL (Seelig et al., 2014; Hughes et al., 2018). In the present work, 25% of the T lymphomas were classified as small clear cell-TZL due to the cytological appearance and the presence of TCD45− lymphocytes by flow cytometry (Seelig et al., 2014; Martini et al., 2016). Although aggressive T lymphomas were prevalent (75%), the importance of the diagnosis of TCD45− lymphoma is based on its better prognosis and less aggressive clinical course (indolent subtype) (Ponce et al., 2004; Valli et al., 2006; Valli et al., 2013; Avery et al., 2014). Little is known about why CD45− T lymphomas are the least aggressive of the lymphomas and the evolution of clinical and paraclinical parameters in patients. CD45 is a T lymphocyte accessory molecule that stabilizes the interaction between T and B cells and plays a vital role in the maturation of the thymocytes (Ledbetter et al., 1993). CD45-null transgenic mice showed a reduced ability to mature CD4− CD8− T cells into CD4+ CD8+ T cells (Byth et al., 1996), which could explain why most patients in the CD45− T cell group were also CD4− CD8− on flow cytometry. Several authors previously reported this aberration (Avery et al., 2014; Seelig et al., 2014; Martini et al., 2015; Pawlak et al., 2016). One-third of all patients with lymphoma had anemia or leukocytosis. Several authors listed anemia as a side effect of chronic disease (Marconato et al., 2013; Valli et al., 2013; Parachini-Winter et al., 2019). Any of the lymphoma groups showed differences in severity. However, dogs with LTCD45− lymphomas had higher leukocyte counts than LTCD45+. Interestingly, patients with CD45− T-cell lymphomas had higher levels of lymphocytes than patients with B-cell and CD45+ T-cell lymphomas, and 60% of TCD45− patients had mature lymphocytosis. Previous studies have shown that mature lymphocytosis is a common finding in indolent TZLs, exceeding 50% of dogs in all cases (Flood-Knapik et al., 2013; Avery et al., 2014; Seelig et al., 2014; Martini et al., 2016). The lack of significant difference in leukocyte count with the LB group was possibly due to the low number of animals in the LTCD45− group. Due to the lack of TCD45, knockout mice showed an increase in splenic B cells, mainly of the B1 population (Byth et al., 1996). In addition, it has been reported that CD45− cells could evade, at least in part, the process of apoptosis, reaching a longer lifespan (Hernandez and Baum, 2002; Seelig et al., 2014; Martini et al., 2016). The highest proportion of eosinopenia was found in CD45− T lymphomas. To the authors’ knowledge, there are no reports of eosinopenia in lymphomas, although there are reports of eosinophilia in intestinal T lymphomas related to paraneoplastic IL-5 synthesis (Marchetti et al., 2005; Ozaki et al., 2006). However, monocytic cell counts were higher than those of CD45+ T lymphomas. It was previously reported that dogs with lymphoma had higher monocyte counts than healthy animals (Perry et al., 2011; Calvalido et al., 2016). However, to the authors’ knowledge, this would be the first study to analyze monocyte counts among T-cell lymphoma subtypes. Despite not being statistically significant, total protein levels were striking higher in patients with LTCD45−. Possible reasons for the lack of significant effect of total protein in our study are a small number of animals analyzed in the LTCD45− group. Albumin and alpha-1 and alpha-2 globulin levels did not differ between the groups. However, gamma globulin levels in LTCD45− were higher than in the other lymphoma groups. Although this finding may be paradoxical, it has been described in several B- and T-lymphocyte disorders in humans (Mims, 2018). This profile could be related to the differences found in circulating lymphocyte levels and the role of CD45 in regulating the immune system. However, few reports describe variations in protein fractions, and none of them consider immunophenotype (Gavazza et al., 2009; Tappin et al., 2011). A patient’s survival depends on the type of lymphoma, the degree of disease progression, and the treatment. Although this work has the limitation of not having standardized treatments among lymphoma immunophenotypes, OST differs between groups. LTCD45− dogs survived longer than the other groups (mean 641 days), while LB dogs (mean 166 days) had a longer survival time than LTCD45+ (62 days) (Fig. 3). The lack of CD45 co-stimulatory T molecules and the potential secondary stimulation of innate responses could explain, at least in part, the low aggressiveness and their classification as indolent T lymphomas. Of note, the OST of our dogs with lymphoma was lower than that reported in the literature (Ruslander et al., 1997; Ponce et al., 2004; Valli et al., 2013; Curran and Thamm, 2016). This could be explained by the fact that many dogs with aggressive lymphoma were not treated or were treated with an inadequate protocol. High-grade T lymphomas are the most aggressive. T cells (CD4+ and CD8+) play an important role in the coordination of the immune system, and their deficiency could severely affect the patient’s health. Hypercalcemia and elevated plasma LDH levels were found in patients severely affected by aggressive T lymphomas (TCD45+). Hypercalcemia is a metabolic disorder frequently associated with advanced malignant disease and was found only in patients with phenotype T (Ruslander et al., 1997; Marconato et al., 2011; Aresu et al., 2015; Harris et al., 2020). Hypercalcemia is due to the production of a peptide (parathyroid hormone related peptide) by neoplastic CD4 T lymphocytes, related to parathyroid hormone (Bryan, 2016; Goldner, 2016). On the other hand, increased LDH levels could correspond to a “shift” toward anaerobic metabolism (increased anaerobic glycolysis), necessary to maintain rapid tumor growth (Fantin et al., 2006). In this regard, previous work found a positive correlation between LDH and the severity of anemia in patients with T-cell lymphoma (Sánchez-Solé, 2019). In this work, the highest values of this enzyme were observed in B-cell lymphomas; however, unlike reported by other authors (Marconato et al., 2013; Marconato et al., 2019), they were not associated with lower survival. Interestingly, in the present work, we found a direct relationship between high LDH values (greater than 500 IU/l) and lower survival in aggressive CD45+ T lymphomas. To the author’s knowledge, this is the first time this relationship has been reported. ConclusionSurvival time and hematologic and biochemical patterns differed among the immunophenotypes of canine lymphomas. Patients of LTCD45− phenotype showed higher lymphocyte counts and gamma globulin levels and more prolonged survival. Further study with a more significant number of LTCD45− dogs is necessary to investigate the role of CD45 molecule in lymphoma pathogenesis, development of paraneoplastic syndromes, and prognosis. Serum LDH activity may provide additional prognostic information in CD45+ T-cell lymphoma. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionRosina Sanchez conducted the research trials, wrote the article, and analyzed the data. Florencia Mosquillo assisted in the methodological development of the flow cytometry. Paulo Jark corrected the final version of the manuscript. Martín Breijo and Paula Pessina designed and supervised the research trials, and assisted in writing and editing the article. AcknowledgmentsThe authors gratefully acknowledge the support of Juan Pablo Damián in the statistical analysis. ReferencesAbbo, A.H. and Lucroy, M.D. 2007. Assessment of anemia as an independent predictor of response to chemotherapy and survival in dogs with Lymphoma: 96 cases (1993-2006). J. Am. Vet. Med. Assoc. 231(12), 1836–1842. Álvarez-Berger, F.J., Aburto, E., Aristi, G. and Chávez, G. 2009. Histological and immunophenotypic study of canine lymphoma in the center of Mexico. Vet. Méx. 40(2), 141–155. Aniolek, O., Gajewski, Z. and Ginzinski, S. 2014. Application of flow cytometry in diagnosing lymphomas in dogs and cats. Centr. Eur. J. Immunol. 39(3), 327–330. Aresu, L., Martini, V., Rossi, F., Vignoli, M., Sampaolo, M., Aricom A., Laganga, P., Pierini, A., Frayssinet, P., Mantovani, R. and Marconato, L. 2015. Canine indolent and aggressive lymphoma: clinical spectrum with histologic correlation. Vet. Comp. Oncol. 13(4), 348–362. Avery, P.R., Burton, J., Bromberek, J.L., Seelig, D.M., Elmslie, R., Correa, S., Ehrhart, E.J., Morley, P.S. and Avery, A.C. 2014. Flow cytometric characterization and clinical outcome of CD4+ T-cell Lymphoma in dogs: 67 Cases. J. Vet. Intern. Med. 28(2), 538–546. Beaver, L.M., Strottner, G. and Klein, M.K. 2010. Response rate after administration of a single dose of doxorubicin in dogs with B-cell or T-cell lymphoma: 41 cases (2006–2008). J. Am. Vet. Med. Assoc. 237(9), 1052–1055. Bergman, P.J. 2012. Paraneoplastic hypercalcemia. Top. Companion Anim. Med. 27(4), 156–158. Breen, M. and Modiano, J.F. 2008. Evolutionarily conserved cytogenetic changes in hematological malignancies of dogs and humans – man and his best friend share more than companionship. Chromosome Res. 16(1),145–154. Bryan, J.N. 2016. The current state of clinical application of serum biomarkers for canine lymphoma. Front. Vet. Sci. 3, 87. Byth, K.F., Conroy, L.A., Howlett, S., Smith, A.J., May, J., Alexander, D.R. and Holmes, N. 1996. CD45-null transgenic mice reveal a positive regulatory role for CD45 in early thymocyte development, in the selection of CD4+CD8+ thymocytes, and B cell maturation. J. Exp. Med. 183(4), 1707–1718. Calvalido, J., Wood, G., Mutsaers, A., Wood, D., Sears, W. and Wood, J.P. 2016. Comparison of serum cytokine levels between dogs with multicentric lymphoma and healthy dogs. Vet. Inmunol. Inmunopathol. 182, 106–112. Comazzi, S., Marelli, S., Cozz, M., Rizzi, R., Finotello, R., Henriques, J., Pastor, J., Ponce, F., Rohrer-Bley, C., Rütgen, B. and Teske, E. 2018. Breed-associated risks for developing canine lymphoma differ among countries: an European canine lymphoma network study. BMC Vet. Res. 14(1), 232. Curran, K. and Thamm, D.H. 2016. Retrospective analysis for treatment of naïve canine multicentric lymphoma with a 15-week, maintenance-free CHOP protocol. Vet. Comp. Oncol. 14(1), 147–155. Dorn, C.R., Taylor, D.O. and Hibbard, H.H. 1967. Epizootiologic characteristics of canine and feline leukemia and lymphoma. Am. J. Vet. Res. 28(125), 993–1001. Ernst, T., Kessler, M., Lautscham, E., Willimzig, L. and Neiger, R. 2016. Das multizentrische Lymphom bei 411 Hunden–eine epidemiologische Studie. Tierarztl. Prax. Ausg. K. Kleintiere. Heimtiere. 44(4), 245–251. Fantin, V.R., St-Pierre, J. and Leder, P. 2006. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 9(6), 425–434. Ferrari, A., Cozzi, M., Aresu, L. and Martini, V. 2021. Tumor staging in a Beagle dog with concomitant large B-cell lymphoma and T-cell acute lymphoblastic leukemia. J. Vet. Diagn. Invest. 33(4), 792–796. Flood-Knapik, K.E., Durham, A.C., Gregor, T.P., Sánchez, M.D., Durney, M.E. and Sorenmo, K.U. 2013. Clinical, histopathological and immunohistochemical characterization of canine indolent lymphoma. Vet. Comp. Oncol. 11(4), 272–286. Fontaine, S.J., McCulloch, E., Eckersall, P.D., Haining, H., Patterson Kane, J.C. and Morris, J.S. 2017. Evaluation of the modified Glasgow Prognostic Score to predict outcome in dogs with newly diagnosed lymphoma. Vet. Comp. Oncol. 15(4), 1513–1526. Fournel-Fleury, C., Magnol, J.P., Bricaire, P., Marchal, T., Chabanne, L., Delverdier, A., Bryon, P.A. and Felman, P. 1997. Cytohistological and immunological classification of canine malignant lymphomas: comparison with human non-Hodgkin’s lymphomas. J. Comp. Pathol. 117(1), 35–59. Gavazza, A., Lubas, G., Valori, E. and Gugliucci, B. 2008. Retrospective survey of malignant lymphoma cases in the dog: clinical, therapeutical and prognostic features. Vet. Res. Commun. 32, 291–293. Gavazza, A., Sacchini, F., Lubas, G., Gugliucci, B. and Valori, E. 2009. Clinical, laboratory, diagnostic and prognostic aspects of canine lymphoma: a retrospective study. Comp. Clin. Pathol. 18, 291–299. Goldner, W. 2016. Cancer-related hypercalcemia. J. Oncol. Pract. 12(5), 426–432. Harris, L.J., Rout, E.D., Labadie, J.D., Avery, P.R., Fernandez, M., Yoshimoto, J. and Avery, A.C. 2020. Clinical features of canine nodal T-cell lymphomas classified as CD8+ or CD4−CD8− by flow cytometry. Vet. Comp. Oncol. 18(3), 416–427. Hernandez, J.D. and Baum, L.G. 2002. Ah, sweet mystery of death! Galectins and control of cell fate. Glycobiology 12(10), 127R–136R. Hughes, K.L., Labadie, J.D., Yoshimoto, J.A., Dossey, J.J., Burnett, R.C. and Avery, A.C. 2018. Increased frequency of CD45 negative T cells (T zone cells) in older Golden retriever dogs. Vet. Comp. Oncol. 16(1), E109–E116. Jalla, S., Sazawal, S., Deb, S., Black, R.E., Das, S.N., Sarkar, A. and Bhan, M., 2004. Enumeration of lymphocyte subsets using flow cytometry: effect of storage before and after staining in a developing country setting. Indian J. Clin. Biochem. 19(2), 95–99. Jark, P.C., Fracacio, P., Anai, L.A., Silva, M.C.L., Calazans, S.G., Senhorello, I.L.S., Costa, M.T., Sequeira, J.L. and Sueiro, F.A.R. 2020. Histopathological and immunophenotypical characterization of canine multicentric Lymphoma in Brazil: a study of 203 cases. Arq. Bras. Med. Vet. Zootec. 72(3), 787–793. Kiupel, M., Teske, E. and Bostock, D. 1999. Prognostic factors for treated canine malignant lymphoma. Vet. Pathol. 36(4), 292–300. Lana, S.E., Plaza, S., Hampe, K., Burnett, R.C. and Avery, A.C. 2006. Diagnosis of mediastinal masses in dogs by flow cytometry. J. Vet. Intern. Med. 20, 1161–1165. Ledbetter, J.A., Deans, J.P., Aruffo, A., Grosmaire, L.S., Kanner, S.B., Bolen, J.B. and Schieven, G.L. 1993. CD4, CD8 and the role of CD45 in T-cell activation. Curr. Opin. Immunol. 5(3), 334–340. Marchetti, V., Benetti, C., Citi, S. and Taccini, V. 2005. Paraneoplastic hypereosinophilia in a dog with intestinal T-cell lymphoma. Vet. Clin. Pathol. 34(3), 259–263. Marconato, L., Leo, C., Girelli, R., Salvi, S., Abramo, F., Bettini, G., Comazzi, S., Nardi, P., Albanese, F. and Zini, E. 2009. Association between waste management and cancer in companion animals. J. Vet. Intern. Med. 23(3), 564–569. Marconato, L., Crispino, G., Finotello, R., Mazzotti, S. and Zini, E., 2010. Clinical relevance of serial determinations of lactate dehydrogenase activity used to predict recurrence in dogs with lymphoma. J. Am. Vet. Med. Assoc. 236(9), 969–974. Marconato, L., Stefanello, D., Valenti, P., Bonfanti, U., Comazzi, S., Roccabianca, P., Caniatti, M., Romanelli, G., Massari, F. and Zini, E. 2011. Predictors of long-term survival in dogs with high grade multicentric lymphoma. J. Am. Vet. Med. Assoc. 238(4), 480–485. Marconato, L., Martini, V., Aresu, L., Sampaolo, M., Valentini, F., Rinaldi, V. and Comazzi, S. 2013. Assessment of bone marrow infiltration diagnosed by flow cytometry in canine large B cell lymphoma: prognostic significance and proposal of a cut-off value. Vet. J. 197(3), 776–781. Marconato, L., Comazzi, S., Aresu, L., Riondato, F., Stefanello, D., Ferrari, R. and Martini V. 2019. Prognostic significance of peripheral blood and bone marrow infiltration in newly-diagnosed canine nodal marginal zone lymphoma. Vet. J. 246, 78–84. Martini, V., Poggi, A., Riondato, F., Gelain, M. E., Aresu, L. and Comazzi, S. 2015. Flow-cytometric detection of phenotypic aberrancies in canine small clear cell lymphoma. Vet. Comp. Oncol. 13(3), 281–287. Martini, V., Marconato, L., Poggi, A., Riondato, F., Aresu, L., Cozzi, M. and Comazzi, S. 2016. Canine small clear cell/T-zone lymphoma: clinical presentation and outcome in a retrospective case series. Vet. Comp. Oncol. 14(1), 117–126. Martini, V., Cozzi, M., Aricò, A., Dalla Rovere, G., Poggi, A., Albonico, F., Mortarino, M., Ciusani, E., Aresu, L. and Comazzi, S. 2017. Loss of CD45 cell surface expression in canine T-zone lymphoma results from reduced gene expression. Vet. Immunol. Immunopathol. 187, 14–19. Martini, V., Bernardi, S., Giordano, A. and Comazzi, S. 2020. Flow cytometry expression pattern of CD44 and CD18 markers on feline leukocytes. J. Vet. Diagn. Invest. 32(5), 706–709. Merlo, D.F., Rossi, L., Pellegrino, C., Ceppi, M., Cardellino, U., Capurro, C., Ratto, A., Sambucco, P.L., Sestito, V., Tanara, G. and Bocchini, V. 2008. Cancer incidence in pet dogs: findings of the animal tumor registry of Genoa, Italy. J. Vet. Intern. Med. 22(4), 976–984. Miller, A.G., Morley, P.S., Rapo, S., Avery, A.C., Lana, S.E. and Olver, C.S. 2009. Anemia is associated with decreased survival time in dogs with lymphoma. J. Vet. Intern. Med. 23(1), 116–122. Mims, M.P. 2018. Lymphocytosis, lymphocytopenia, hypergammaglobulinemia, and hypogammaglobulinemia. In Hematology, basic principles and practice, Eds., Hoffman, R., Benz, E.J., Silbrstein, L.E., Heslop, H.E., Weitz, J.I., Anastasi, J., Salama, M.E. and Abutalib, S.A. Philadelphia, PL: Elsevier, pp: 682–690. Modiano, J.F., Breen, M., Burnett, R.C., Parker, H.G., Inusah, S., Thomas, R.,Avery, P.R., Lindblad-Toh, K., Ostrander, E.A., Cutter, G.C. and Avery, A.C. 2005. Distinct B-cell and T-cell lymphoproliferative disease prevalence among dog breeds indicates heritable risk. Cancer Res. 65(13), 5654–5661. Moreno, K. and Bracarense, A. 2007. Linfoma canino de células T: aspectos epidemiológicos, clínicos e morfológicos de 38 casos. Braz. J. Vet. Anim. Sci. 44, 103–110. Neuwald, E.B., Teixeira, L.V., Conrado, F.O., da Silva, M.O.D., Hlavac, N.R.C. and González, F.H.D. 2014. Epidemiological, clinical and immunohistochemical aspects of canine lymphoma in the region of Porto Alegre, Brazil. Pesq. Vet. Bras. 34(4), 349–354. Ozaki, K., Yamagami, T., Nomura, K. and Namara, I. 2006. T-cell lymphoma with eosinophilic infiltration involving the intestinal tract in 11 dogs. Vet. Pathol. 43(3), 339–344. Papakonstantinou, S., Berzina, I., Lawlor, A., O’Neill, E.J. and O’Brien, P.J. 2013. Rapid, effective and user-friendly immunophenotyping of canine lymphoma using a personal flow cytometer. Ir. Vet. J. 66(1), 6. Parachini-Winter, C., Carioto, L.M. and Gara-Boivin, C. 2019. Retrospective evaluation of anemia and erythrocyte morphological anomalies in dogs with lymphoma or inflammatory bowel disease. J. Am. Vet. Med. Assoc. 254(4), 487–495. Pastor, M., Chalvet-Monfray, K., Marchal, T., Keck, G., Magnol, J.P., Fournel-Fleury, C. and Ponce, F. 2009. Genetic and environmental risk indicators in canine non-Hodgkin’s lymphomas: breed associations and geographic distribution of 608 cases diagnosed throughout France over 1 year. J. Vet. Intern. Med. 23(2), 301–310. Pawlak, A., Rapak, A., Drynda, A., Poradowski, D., Zbyryt, I., Dzimira, S., Suchanski, J. and Obminska-Mrukowicz, B. 2016. Immunophenotypic characterization of canine malignant lymphoma: a retrospective study of cases diagnosed in Poland Lower Silesia, over the period 2011–2013. Vet. Comp. Oncol. 14(1), 52–60. Perry, J.A., Thamm, D.H., Eickhoff, J., Avery, A.C. and Dow, S.W. 2011. Increased monocyte chemotactic protein-1 concentration and monocyte count independently associate with a poor prognosis in dogs with lymphoma. Vet. Comp. Oncol. 9(1), 55–64. Ponce, F., Magnol, J.P., Ledieu, D., Marchal, T., Turinelli, V., Chalvet-Monfray, K. and Fournel-Fleury, C. 2004. Prognostic significance of morphological subtypes in canine malignant lymphomas during chemotherapy. Vet. J. 167(2), 158–166. Ponce, F., Marchal, T., Magnol, J.P., Turinelli, V., Ledieu, D., Bonnefont, C., Pastor, M., Delignette, M.L. and Fournel-Fleury, C. 2010. A morphological study of 608 cases of canine malignant lymphoma in France with a focus on comparative similarities between canine and human lymphoma morphology. Vet. Pathol. 47(3), 414–433. Ruple, A., Avery, A.C. and Morley, P.S. 2017. Differences in the geographic distribution of lymphoma subtypes in Golden retrievers in the USA. Vet. Comp. Oncol. 15(4), 1590–1597. Ruslander, D.A., Gebhard, D.H., Tompkins, M.B., Grindem, C.B. and Page, R.L. 1997. Immunophenotypic characterization of canine lymphoproliferative disorders. In Vivo (Athens, Greece). 11(2), 169–172. Sánchez-Solé, R. 2019. Linfoma canino: clasificación inmunofenotípica y su relación con patrones hematológicos, bioquímicos y moleculares, M.S. thesis. Montevideo, Uruguay: Universidad de la República. Seelig, D.M., Avery, P., Webb, T., Yoshimoto, J., Bromberek, J., Ehrhart, E.J. and Avery, A.C. 2014. Canine T-zone lymphoma: unique immunophenotypic features, outcome, and population characteristics. J. Vet. Intern. Med. 28(3), 878–886. Tappin, S.W., Taylor, S.S., Tasker, S., Dodkin, S.J., Papasouliotis, K. and Murphy, K.F. 2011. Serum protein electrophoresis in 147 dogs. Vet. Rec. 168(17), 456. Teske, E., de Vos, J.P., Egberink, H.F. and Vos, J.H., 1994. Clustering in canine malignant lymphoma. Vet. Q. 16(2), 134–136. Vail, D.M., Pinkerton, M.E. and Young, K.M. 2013. Hematopoietic tumors. In Small animal clinical oncology, Eds., Withrow and MacEwen’s. 5th ed. St.Louis, MI: Elsevier, pp: 608–677. Valli, V.E., Vernau, W., de Lorimier, L.P., Graham, P.S. and Moore, P.F. 2006. Canine indolent nodular lymphoma. Vet. Pathol. 43(3), 241–256. Valli, V.E., San Myint, M., Barthel, A., Bienzle, D., Caswell, J., Colbatzky, F., Durham, A., Ehrhart, E.J., Johnson, Y., Jones, C., Kiupel, M., Labelle, P., Lester, S., Miller, M., Moorem P., Moroff, S., Roccabianca, P., Ramos-Vara, J., Ross, A., Scase, T., Tvedten, H. and Vernau, W. 2011. Classification of canine malignant lymphomas according to the World Health Organization criteria. Vet. Pathol. 48(1), 198–211. Valli, V.E., Kass, P.H., Myint, M.S. and Scott, F. 2013. Canine lymphomas: association of classification type, disease stage, tumor subtype, mitotic rate, and treatment with survival. Vet. Pathol. 50(5), 738–748. Zanatta, R., Abate, O., D’Angelo, A., Miniscalco, B. and Mannelli, A. 2003. Diagnostic and prognostic value of serum lactate dehydrogenase (LDH) and LDH isoenzymes in canine lymphoma. Vet. Res. Commun. 27, 449–452. | ||
| How to Cite this Article |
| Pubmed Style Solé RS, Mosquillo F, Jark P, Breijo M, Pessina P. Hematological and biochemical profiles of canine CD45- T lymphomas are different from other immunophenotypes. Open Vet. J.. 2021; 11(4): 734-746. doi:10.5455/OVJ.2021.v11.i4.26 Web Style Solé RS, Mosquillo F, Jark P, Breijo M, Pessina P. Hematological and biochemical profiles of canine CD45- T lymphomas are different from other immunophenotypes. https://www.openveterinaryjournal.com/?mno=121468 [Access: January 25, 2026]. doi:10.5455/OVJ.2021.v11.i4.26 AMA (American Medical Association) Style Solé RS, Mosquillo F, Jark P, Breijo M, Pessina P. Hematological and biochemical profiles of canine CD45- T lymphomas are different from other immunophenotypes. Open Vet. J.. 2021; 11(4): 734-746. doi:10.5455/OVJ.2021.v11.i4.26 Vancouver/ICMJE Style Solé RS, Mosquillo F, Jark P, Breijo M, Pessina P. Hematological and biochemical profiles of canine CD45- T lymphomas are different from other immunophenotypes. Open Vet. J.. (2021), [cited January 25, 2026]; 11(4): 734-746. doi:10.5455/OVJ.2021.v11.i4.26 Harvard Style Solé, R. S., Mosquillo, . F., Jark, . P., Breijo, . M. & Pessina, . P. (2021) Hematological and biochemical profiles of canine CD45- T lymphomas are different from other immunophenotypes. Open Vet. J., 11 (4), 734-746. doi:10.5455/OVJ.2021.v11.i4.26 Turabian Style Solé, Rosina Sánchez, Florencia Mosquillo, Paulo Jark, Martn Breijo, and Paula Pessina. 2021. Hematological and biochemical profiles of canine CD45- T lymphomas are different from other immunophenotypes. Open Veterinary Journal, 11 (4), 734-746. doi:10.5455/OVJ.2021.v11.i4.26 Chicago Style Solé, Rosina Sánchez, Florencia Mosquillo, Paulo Jark, Martn Breijo, and Paula Pessina. "Hematological and biochemical profiles of canine CD45- T lymphomas are different from other immunophenotypes." Open Veterinary Journal 11 (2021), 734-746. doi:10.5455/OVJ.2021.v11.i4.26 MLA (The Modern Language Association) Style Solé, Rosina Sánchez, Florencia Mosquillo, Paulo Jark, Martn Breijo, and Paula Pessina. "Hematological and biochemical profiles of canine CD45- T lymphomas are different from other immunophenotypes." Open Veterinary Journal 11.4 (2021), 734-746. Print. doi:10.5455/OVJ.2021.v11.i4.26 APA (American Psychological Association) Style Solé, R. S., Mosquillo, . F., Jark, . P., Breijo, . M. & Pessina, . P. (2021) Hematological and biochemical profiles of canine CD45- T lymphomas are different from other immunophenotypes. Open Veterinary Journal, 11 (4), 734-746. doi:10.5455/OVJ.2021.v11.i4.26 |