| Original Article | ||
Open Vet. J.. 2021; 11(4): 686-694 Open Veterinary Journal, (2021), Vol. 11(4): 686–694 Original Research Peripheral nerve regeneration: A comparative study of the effects of autologous bone marrow-derived mesenchymal stem cells, platelet-rich plasma, and lateral saphenous vein graft as a conduit in a dog modelMousa H. Daradka*, Zuhair A. Bani Ismail and Mohammad A. IrsheidDepartment of Veterinary Clinical Sciences, Faculty of Veterinary Medicine, Jordan University of Science and Technology, Irbid, Jordan *Corresponding Author: Mousa H. Daradka. Department of Veterinary Clinical Sciences, Faculty of Veterinary Medicine, Jordan University of Science and Technology, Irbid, Jordan. Email: daradka [at] just.edu.jo Submitted: 13/09/2021 Accepted: 11/11/2021 Published: 05/12/2021 © 2021 Open Veterinary Journal
AbstractBackground: The quality of healing of peripheral nerve injuries remains a common challenge causing pain and poor quality of life for millions of people and animals annually. Aims: The objectives of this study were to evaluate the healing quality of facial nerve injury in a dog model following local treatment using an autologous injection of platelet-rich plasma (PRP) or bone marrow-derived mesenchymal stem cells (BM-MSCs) at the injury site in combination with the application of an autologous saphenous vein graft as a conduit. Methods: 20 apparently healthy adult Mongrel dogs were randomly divided into 4 equal groups. Dogs in groups 1, 2, and 3 were subjected to facial nerve neurectomy and saphenous vein conduit graft implantation at the site of facial nerve injury. Dogs in groups 2 and 3 received 1 ml of autologous PRP and BM-MSCs, respectively. Injections were administered directly in the vein conduit immediately after nerve injury. Dogs in group 1 (grafted but not treated; control) received only an autologous vein graft, and those in group 4 (normal control) received no graft and no PRP or BM-MSCs treatment. The dogs were monitored daily for 8 weeks after surgery. Clinical evaluation of the facial nerve, including lower eyelid, ear drooping, upper lip, and tongue functions, was carried out once per week using a numerical scoring system of 0–3. At the end of the study period (week 8), the facial nerve injury site was evaluated grossly for the presence of adhesions using a numerical scoring system of 0–3. The facial nerve injury site was histopathologically assessed for the existence of perivascular mononuclear cell infiltration, fibrous tissue deposition, and axonal injury using H&E-stained tissue sections. Results: Clinically, BM-MSCs treated dogs experienced significant (p < 0.05) improvement in the lower eyelid, ear, lip, and tongue functions 4 weeks postoperatively compared to other groups. Grossly, the facial nerve graft site in the BM-MSCs treated group showed significantly (p < 0.05) lesser adhesion scores than the other groups. Histopathologically, there was significantly (p < 0.05) less perivascular mononuclear cell infiltration, less collagen deposition, and more normal axons at the facial nerve injury site in the BM-MSCs treated group compared to the other groups. Conclusion: This study showed clinically significant enhancement of nerve regeneration by applying autologous BM-MSCs and autologous vein grafting at the site of facial nerve injury. However, further clinical trials are warranted before this application can be recommended to treat traumatic nerve injuries in the field. Keywords: Neuropathies, Pain, Nerve regeneration, Pluripotent cells, Nerve graft. IntroductionBone marrow-derived mesenchymal stem cells (BM-MSCs) are pluripotent progenitor cells capable of undergoing differentiation into different cells (Khan and Newsome, 2019). In nervous tissues, BM-MSCs have been shown to promote structural and functional nerve regeneration by differentiating into Schwann cell-like cells and inducing the production of local neurotrophic growth factors necessary for axonal growth and conversion of stem cells into myelinating cells (Cooney et al., 2016). Platelet-rich plasma (PRP), on the other hand, is known to contain high concentrations of many neurotrophic growth factors that enhance the healing of injured nerve tissues, such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), fibrin, fibronectin, and vitronectin (Arnoczky et al., 2011; Wasterlain et al., 2012). Furthermore, it has been shown that PRP provides neuroprotection against cell apoptosis, stimulates angiogenesis, enhances axonal regeneration and growth, and improves the local microenvironment by overcoming inflammatory responses (Sanchez et al., 2017). Peripheral nerve injuries (PNIs) remain a common challenge in both human and veterinary medicine, causing pain and poor quality of life for millions of people and animals annually (Martinez et al., 2011; Daly et al., 2012). Unlike healing of nerve tissues in the central nervous system, the regeneration of the peripheral nervous system is considerably more efficient (Gaudet et al., 2011). Unfortunately, this remarkable healing ability is often hampered by significant nerve gaps due to traumatic injuries (Gaudet et al., 2011). The sural nerve graft remains the most common autograft donor that is used to bridge nerve gaps induced by injuries (Ryu et al., 2011). Other methods such as bio-tubes and conduits were also used previously (Sinis et al., 2011). Conduits, which are tubes made of silicone, have been demonstrated to induce rapid functional recovery and enhance microscopic healing when used in nerve reconstruction of acute, subacute, and chronic injuries (Sinis et al., 2011). Recent studies using autologous vein grafts have shown significant improvement in the healing of injured nerves with less formation of fibrous tissues and adhesions (Mathieu et al., 2012). In this study, an autologous vein graft harvested from the lateral saphenous vein was used for the first time as a conduit for the treatment of PNI of the facial nerve using either BM-MSCs or PRP in a dog model. Materials and MethodsAnimalsA total of 20 apparently healthy adult Mongrel dogs weighing between 15 and 20 kg were used in the study. The dogs were purchased from a local breeder and allowed 2 weeks of acclimatization. Before the analysis, the dogs were subjected to a complete general physical examination, including neurological evaluation. Special attention was paid to evaluating facial expression and cranial nerve functions, including masseter muscles symmetry, size, and tone, palpebral reflex, corneal examination, tongue tone and function, and nostril and labial symmetry and function. In addition, complete blood cell count and serum chemistry profile were carried out for all animals. Only dogs with normal findings were allowed to be enrolled in the study. During the acclimatization period, the dogs were treated for internal parasites (Ascaten; Alfasan, Holland) with 1 tablet per 5 kg and vaccinated against canine distemper virus, canine adenovirus type 1, canine adenovirus type 2, canine parainfluenza virus, canine parvovirus (Vanguard Plus 5, Zoetis, USA), and Leptospira spp. (MSD Animal Health, Ireland). The dogs were identified using neck collars and housed individually in cages. Dogs were fed twice daily using locally produced dog pelleted feed and offered fresh drinking water ad-lib. Study designThe dogs were randomly divided into four equal groups (five dogs each). Dogs in groups 1, 2, and 3 were subjected to facial nerve neurectomy and saphenous vein conduit graft implantation at the site of facial nerve injury. Dogs in groups 2 and 3 received 1 ml of autologous PRP and BM-MSCs, respectively. Injections were administered directly in the vein conduit immediately after nerve injury was created. Dogs in group 1 (grafted but not treated; control) received only an autologous vein graft. Those in group 4 (normal control) received no facial nerve neurectomy, vein graft, and injections. All dogs were monitored daily for 8 weeks after surgery. Anesthesia and analgesiaThe dogs were fasted for 12 hours, and water was withheld for 6 hours before the induction of general anesthesia. General anesthesia was induced by intramuscular injection of xylazine (Xylaject 20%; Adwia, Egypt) at 1.1 mg/kg and ketamine (Ketamine HCl 10%; Alfasan, Holland) at 5 mg/kg. Maintenance of anesthesia was achieved by repeated injections of xylazine and ketamine, intramuscularly as needed. Intravenous isotonic fluids were administered during the entire time the dogs were maintained under general anesthesia at 10 ml/kg/hour. PRP preparationAutologous PRP was collected and prepared according to previously described methods (Daradka et al., 2016). Briefly, approximately 20 ml of whole blood was collected aseptically via cephalic vein puncture using vacutainer needles and placed immediately in ethylenediamine tetraacetic acid-containing tubes. The PRP was collected using double centrifugation cycles at 200 and 400 g at 22°C for 10 minutes, respectively. The number of platelets in PRP was then adjusted to approximately 5 × 106/ml using sterile phosphate-buffered saline (PBS) and used immediately to treat the nerve injury. Bone marrow aspiration and stem cell preparationBone marrow aspiration was carried out 2 weeks before creating facial nerve injury to allow adequate time for stem cell preparation. With the dogs under general anesthesia, bone marrow aspiration was carried out according to previously described methods with modifications (Muir et al., 2016). Approximately 20 ml of bone marrow was aspirated using an 18-gauge needle attached to a 20 ml syringe containing traces of heparin from the iliac crest. Collected bone marrow was transferred to the laboratory immediately to isolate the stem cells according to previously described methods (Somasundaram et al., 2015). Briefly, approximately 40 ml of sterile PBS was added to 20 ml of bone marrow aspirate and placed in glass tubes containing Ficoll-Paque solution (Merck, Germany). The glass tubes were then centrifuged and washed twice using PBS at 400 g for 10 minutes. The mononuclear cell layer (buffy coat) was aspirated and rewashed using PBS to remove Ficoll-Paque residues. After that, the erythrocytes portion in the mononuclear cell suspension was removed by lysis using 0.7% NH4Cl solution for 3 minutes. The lysis reaction was abruptly stopped by 0.9% ice-cold NaCl solution. The mononuclear cell suspension was centrifuged again at 400 g for 10 minutes at room temperature. The cell pellet was suspended in Minimum Essential Medium α (Mediatech, USA) containing 10% fetal bovine serum, 1× nonessential amino acids, 4 mM L-glutamine, 100 U/ml penicillin, and 0.01 mg/ml streptomycin sulfate (Life Technologies, USA). The media was left at room temperature for 24 hours and nonadhered cells were removed by changing the media. The media was cultured at 37°C under 5% CO2 and 95% humidity for 1 week. The bone marrow cells were then used for the autologous treatment of the nerve injury. At treatment time, the BM-MSCs were detached from culture flasks using trypLE (Life Technologies, USA), counted using a hemocytometer, and adjusted to 1 × 106 per ml of saline. Each dog in group 3 received 1 ml BM-MSCs at the facial nerve injury site under general anesthesia. Lateral saphenous vein graft harvestingWhile the animal was still under general anesthesia, the lateral side of the right hind leg just above the proximal hock joint was prepared for aseptic surgery. An approximately 3 cm long segment from the right lateral saphenous vein was collected by no-touch technique (Papakonstantinou et al., 2016). Briefly, an approximately 5–6 cm long skin incision was made over the saphenous vein. The skin and soft tissues were dissected and reflected using a self-retaining retractor. After the vein was adequately exposed, 2-0 absorbable suture (Atramat, Mexico) was used to ligate both ends of the proposed section of the vein using double ligation, and the vein was severed between the two ligatures. The collected vein segment was immediately placed in a sterile Petri dish containing cold, sterile saline containing 10% gentamicin. Creation of facial nerve injury, grafting, and treatment protocolThe right side of the face was prepared for aseptic surgery under general anesthesia. An approximately 4–5 cm long vertical skin incision was made anterior to the ear and parallel to the mandibular ramus. The ventral buccal branch of the facial nerve was then carefully dissected and exposed. The nerve was straightened (without tension), and 1 cm long segment was surgically removed using a scalpel blade. After that, the circular autologous saphenous vein graft was placed around the severed nerve ends, covering approximately 0.5 cm proximal and 0.5 cm distal to the cut ends of the nerve. In a simple interrupted pattern, the graft was sutured to the perineurium using 4-0 monofilament suture material. Dogs in groups 1, 2, and 3 received 1 ml saline, 1 ml of PRP, or 1 ml of BM-MSCs, respectively. The treatment was carried out immediately after the nerve injury was created. The treatment solutions (saline, PRP, and BM-MSCs) were injected into the conduit to fill the lumen with the injected material. The subcutaneous tissues and skin were then closed routinely using a simple interrupted pattern and 2-0 absorbable suture material (Atramat; Mexico). Animals in groups 1, 2, and 3 were subjected to saphenous vein harvesting and facial nerve neurectomy, while animals in group 4 were not operated on and received no treatments, and served as normal control. Postoperative careAll animals were allowed to recover from anesthesia under close monitoring in their cages. Dogs were then monitored daily for abnormal clinical signs of fever, anorexia, and depression. The surgical sites, including the saphenous vein collection site and the facial nerve treatment site, were also monitored daily for abnormal local signs such as swelling, pain, or discharge. During the immediate postoperative period (5 days), all animals were treated using amoxicillin (Clamoxyl; Zoetis, USA) at 15 mg/kg orally twice per day and meloxicam (Metacam; Boehringer Ingelheim, Germany) at 0.2 mg/kg once orally. Clinical evaluation of facial nerve functionsAll dogs were subjected to careful evaluation of cranial nerve functions once a day for 8 weeks postoperatively. Any abnormal signs related to facial nerve injury, including eyelid position, lip deviation, degree of salivation, and tongue position, were evaluated using a numerical scoring system of 0–3 (Table 1). The scoring system was developed, pretested, and validated before the study was initiated using three pilot dogs. The evaluation was carried out by a blinded and trained researcher. Gross evaluationAt the end of the observation period (8 weeks), animals were humanely euthanatized using an intravenous anesthetic injection overdose (Leary et al., 2013). Immediately following euthanasia, the animals were subjected to complete necropsy. Special attention was given to examining the site of facial nerve injury. The facial nerve was carefully dissected and inspected. The degree of adhesion formation involving the nerve injury site was assessed using a score of 0–3 where 0 indicates normal tissue, 1 indicates mild adhesion formation, 2 indicates moderate adhesion formation, and 3 indicates extensive adhesion formation. A trained and blinded researcher carried out the necropsy and gross evaluation procedures. Histological evaluationA total of three tissue samples, 0.5 mm in length, were collected from each nerve. One sample from the injury site, including the conduit, one sample from the nerve segment immediately proximal, and one distal to the conduit site to ensure normal tissues were included in the sample. Tissue samples were placed directly in 10% buffered formalin solution and transported to the laboratory for routine histopathological processing. Fixed tissue samples were embedded in paraffin, cut into 5 μm thick sections, and stained using hematoxylin and eosin (H&E) staining. Stained tissue sections were examined by a pathologist who remained blinded to the treatment group using a light microscope with 10× magnification lenses. The severity of cellular infiltration was estimated by counting the total number of perivascular mononuclear cell infiltrates in H&E-stained sections. The degree of cellular infiltrate was then given a score of 0–3 where 0 indicates normal tissue, 1 indicates minimum perivascular cell infiltrate, 2 indicates moderate perivascular cell infiltrate, and 3 indicates severe perivascular cell infiltrate (Table 2). The severity of the axonal injury was assessed using a scale of 0–3 where 0 indicates normal tissue, 1 indicates few scattered injured axons, 2 indicates mild to moderate axonal injury, and 3 indicates scattered severe axonal injury (Table 2). Fibrous tissue formation was assessed by assessing the degree of collagen deposition using a score 0–3 where 0 indicates normal tissue, 1 indicates minimum collagen deposition, 2 indicates moderate collagen deposition, and 3 indicates severe collagen deposition (Table 2). Table 1. Scoring system used to assess clinical signs related to facial nerve injury in dogs.
Table 2. Scoring system used to assess the histopathological features at the site of facial nerve injury in dogs.
Statistical analysisData are presented in median ± standard error. All clinical, gross, and histopathological evaluations were analyzed using the Kruskal–Wallis test followed by the Mann–Whitney test. Statistical analysis was carried out using Statistical Package for the Social Sciences IBM statistical software version 22 (IBM Statistics, USA). Statistical differences were considered significant at p-value < 0.05. Ethical approvalAll experimental procedures used in this study were approved by the Institutional Animal Care and Use Committee of Jordan University of Science and Technology (JUST-ACUC-05-2016). ResultsClinical evaluationSignificant differences (p < 0.05) were detected in the clinical scores of the lower eyelid, ear position, upper lip deviation, degree of salivation, and tongue position in BM-MSCs treated group starting at week 4 and which remained significant throughout the study period. No significant differences were noted between the groups in any clinical parameters during weeks 1–3. Clinically, dogs in the BM-MSCs treated group experienced improved lower eyelid function, less ear drooping, less upper lip deviation, less saliva drooling, and improved tongue tone and position after week 4 in comparison to all other groups. Gross evaluationExamination of the facial nerve injury site at week 8 of the study revealed statistically significant differences (p < 0.05) in the scores of fibrosis and adhesion formation and revascularization in BM-MSCs treated dogs compared to all other groups (Figs. 1 and 2). The facial nerve injury site in BM-MSCs treated group showed significantly less fibrosis and lower adhesion scores, while revascularization was substantially more at this site in this group as compared to other groups. Histological evaluationAnalysis of the histopathological scores at the facial nerve injury site showed statistically significant differences (p < 0.05) between groups regarding inflammatory cell infiltration, fibrous tissue deposition, and continuity of the myelin sheaths. In the BM-MSCs treated group, there was significantly less cellular infiltration and less fibrous tissue deposition with complete continuity of the nerve axon and myelin sheaths compared to the other groups (Figs. 3 and 4). DiscussionTraumatic PNIs cause pain and suffering and represent an excellent treatment challenge (Ciaramitaro et al., 2010). Complete nerve disruption, also called neurotmesis injuries (Sunderland type V), results in total loss of nerve fiber structures and continuity (Caseiro et al., 2016). Generally, these injuries require highly complex reconstructive microsurgery techniques to ensure acceptable results (Gartner et al., 2014; Pereira et al., 2014; Cartarozzi et al., 2015). Although many surgical techniques have been used, including bi-conduits and nerve and vein grafts, healing of severely affected nerves remains suboptimal (Caseiro et al., 2016). Several limitations have been associated with the use of these techniques, including donor and recipient site morbidity in addition to nerve and fiber diameter problems. Therefore, the search for new models and techniques is still widely pursued (Caseiro et al., 2016). In this study, the vein graft did not only provide a scaffold or a conduit to bridge the gap in the injured nerve, but also served as a reservoir where the experimental material was instilled. Although mild inflammatory cell infiltrate at the site of the vein graft was noted in the control group, there was no evidence of tissue rejection. It was clear that the vein graft provided a permeable barrier that allowed transportation of fluids and experimental material (BM-MSCs and PRP) to the surrounding tissues and promoted healing of injured nerves. In addition, it perfectly connected the distal and proximal segments of the transected nerve. The increased cellular infiltrates in the transplanted control group are thought to be part of the normal postsurgical inflammatory reaction and not related to graft rejection or infection.
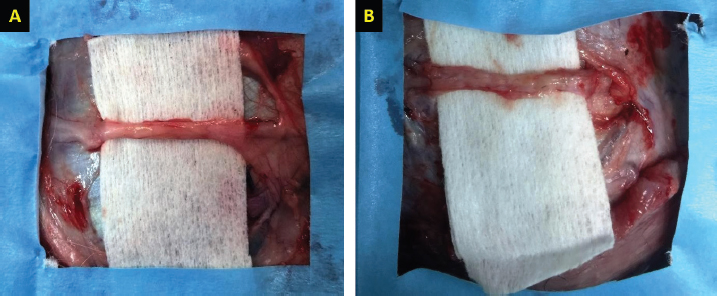
Fig. 1. Gross evaluation of the facial nerve injury site in control groups. Group 1 (A) received an autologous lateral saphenous vein graft only, while dogs in group 4 (B) received no autologous lateral saphenous vein graft, no autologous BM-MSCs and lateral saphenous vein graft, and no platelet-rich plasma. Note the abnormal shape and size of the nerve indicating poor healing with extensive fibrosis.
Fig. 2. Gross evaluation of the facial nerve injury site following treatment using autologous BM-MSCs and lateral saphenous vein graft (A), platelet-rich plasma, and autologous lateral saphenous vein graft (B). Note the significant improvement of nerve shape and size with significantly lower degree of fibrosis and adhesions in group A as compared to other groups.
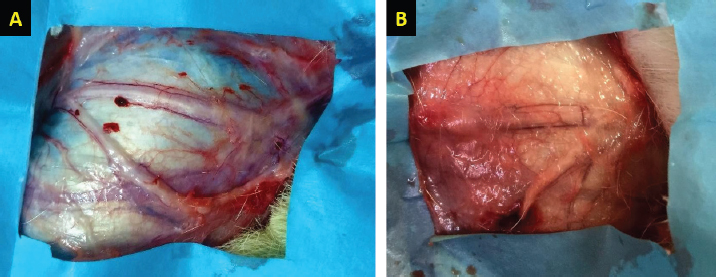
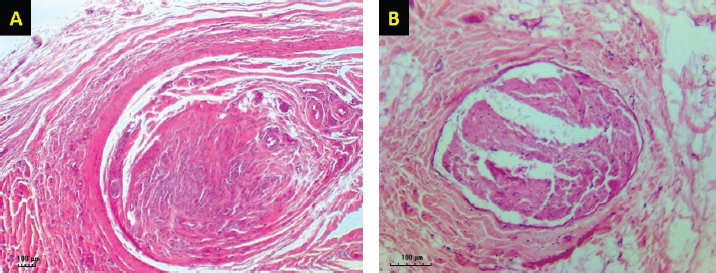
Fig. 3. H&E-stained tissue sections obtained from the facial nerve injury site in control groups. Group 1 (A) received an autologous lateral saphenous vein graft only, while dogs in group 4 (B) received no autologous lateral saphenous vein graft, no autologous BM-MSCs, and no platelet-rich plasma. Note the increased cellular infiltration and deposition of fibrous tissue, scattered and poorly myelinated fibers. (Bar=100 μm).
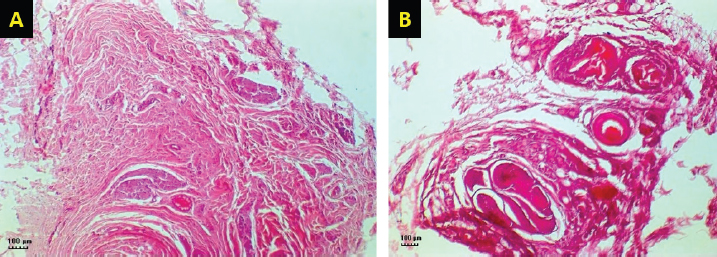
Fig. 4. H&E-stained tissue sections obtained from the facial nerve injury site showing significantly lesser inflammatory cell infiltrates, less fibrous tissue deposition, normal shape and size of the nerve axons, thin but continuum myelin sheaths in BM-MSCs, and lateral saphenous vein graft treated group (A) compared to platelet-rich plasma and autologous lateral saphenous vein graft treated group (B). (Bar=100 μm). Autologous BM-MSCs used in this study showed superior results compared to PRP. BM-MSCs treated animals showed faster and improved clinical recovery of facial nerve functions. These results agree with previous findings in a sciatic nerve transection model in rats treated by the application of MSCs with fibrin scaffold and nerve tubulization (Cartarozzi et al., 2015). It has been reported that BM-MSCs express a neuroprotective function at the injury site by secreting paracrine factors, stimulating cell division, delaying or preventing apoptosis (Nieto et al., 2018). In addition, BM-MSCs promote neurotrophic substances, including NGF, ciliary neurotrophic factor, BDNF, and GDNF, and NGF-B all of which are known to facilitate axon regeneration and remyelination (Nieto et al., 2018). In a previous study, intraneural injection of PRP had improved the functions of the chronically injured radial nerve (de Cortazar et al., 2018). Although the results in this study indicate substantial clinical improvement in PRP-treated dogs compared to the control, the histological evaluation of the nerve injury site showed the presence of a significantly increased fibroblast population. These results are congruent with previously reported findings in the PRP perineural treatment of patients with diabetic neuropathy (Hassanien et al., 2020). In general, the clinical improvement demonstrated in this study was similar in all groups for the first 3 weeks, after which a significant difference was noted in dogs treated by BM-MSCs. Histological evaluation of the BM-MSCs treated group showed the least amount of fibrosis and significantly more normal looking fibers than other groups. These results are similar to previously reported findings (Wang et al., 2015). ConclusionThe results of this study provide a readily accessible alternative to the use of nerve graft for nerve injury treatment. Collection and application of an autologous lateral saphenous vein graft as a conduit to bridge a significant gap in this model of nerve injury and the application of BM-MSCs in the lumen of the grafted vein, which acted as a reservoir, proved to be a valid approach in the management of injured peripheral nerves. However, further clinical studies are warranted to evaluate this approach in treating naturally occurring traumatic PNIs. AcknowledgmentThis study was financially supported by the Deanship of Research at Jordan University of Science and Technology (JUST-ACUC-05-2016). Conflict of interestThe authors declare that they have no competing interests. Authors’ contributionsMousa H. Daradka designed and coordinated all the experimental work. Mohammad A. Irsheid MAI performed the experimental work, handled the animals, collected samples, and drafted the manuscript. Zuhair Bani Ismail analyzed the data, interpreted the results, and edited the manuscript. ReferencesArnoczky, S.P., Delos, D. and Rodeo, S.A. 2011. What is platelet-rich plasma? Oper. Tech. Sports Med. 19(3), 142–148. Cartarozzi, L.P., Spejo, A.B., Ferreira, R.S., Barraviera, B., Duek, E., Carvalho, J.L., Góes, A.M. and Oliveira, A.L. 2015. Mesenchymal stem cells engrafted in a fibrin scaffold stimulate Schwann cell reactivity and axonal regeneration following sciatic nerve tubulization. Brain Res. Bull. 112, 14–24. Caseiro, A.R., Pereira, T., Ivanova, G., Luís, A.L. and Maurício, A.C. 2016. Neuromuscular regeneration: perspective on the application of mesenchymal stem cells and their secretion products. Stem Cells Int. 2016, Article ID:9756973; doi:10.1155/2016/9756973 Ciaramitaro, P., Mondelli, M., Logullo, F., Grimaldi, S., Battiston, B., Sard, A., Scarinzi, C., Migliaretti, G., Faccani, G., Cocito, D. and Italian Network for Traumatic Neuropathies. 2010. Traumatic peripheral nerve injuries: epidemiological findings, neuropathic pain and quality of life in 158 patients. J. Peripher. Nerv. Syst. 15(2), 120–127. Cooney, D.S., Wimmers, E.G., Ibrahim, Z., Grahammer, J., Christensen, J.M., Brat, G.A., Wu, L.W., Sarhane, K.A., Lopez, J., Wallner, C., Furtmüller, G.J., Yuan, N., Pang, J., Sarkar, K., Lee, W.P. and Brandacher, G. 2016. Mesenchymal stem cells enhance nerve regeneration in a rat sciatic nerve repair and hindlimb transplant model. Sci. Rep. 11(6), 31306; doi:10.1038/srep31306 Daly, W., Yao, L., Zeugolis, D., Windebank, A. and Pandit, A. 2012. A biomaterials approach to peripheral nerve regeneration: bridging the peripheral nerve gap and enhancing functional recovery. J. R. Soc. Interface 9(67), 202–221. Daradka, M.H., Malkawi, M.A., Bani Ismail, Z. and Alshehabat, M.A. 2016. Autologous venous grafting promotes healing and reduces adhesion formation in experimentally severed canine superficial digital flexor tendons. Bulg. J. Vet. Med. 19(3), 233–241. de Cortazar, G.U., Padilla, S., Lobato, E., Delgado, D. and Sánchez, M. 2018. Intraneural platelet-rich plasma injections for the treatment of radial nerve section: a case report. J. Clin. Med. 7(2), 13; doi:10.3390/jcm7020013 Gartner, A., Pereira, T., Armada-da-Silva, P.A.S., Amado, S., Veloso, A.P., Amorim, I., Ribeiro, J., Santos, J.D., Bárcia, R.N., Cruz, P., Cruz, H., Luís, A.L., Santos, J.M., Geuna, S. and Maurício, A.C. 2014. Effects of umbilical cord tissue mesenchymal stem cells (UCX) on rat sciatic nerve regeneration after neurotmesis injuries. J. Stem Cells Regen. Med. 10(1), 14–26. Gaudet, A.D., Popovich, P.G. and Ramer, M.S. 2011. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J. Neuroinflammation 8, 110; doi:10.1186/1742-2094-8-110 Hassanien, M., Elawamy, A., Kamel, E.Z., Khalifa, W.A., Abolfad, G.M., Roushdy, A.S., El Zohne, R.A. and Makarem, Y.S. 2020. Perineural platelet-rich plasma for diabetic neuropathic pain, could it make a difference? Pain Med. 21(4), 757–765. Khan, R.S. and Newsome, P.N. 2019. A comparison of phenotypic and functional properties of mesenchymal stromal cells and multipotent adult progenitor cells. Front. Immunol. 10, 1952; doi:10.3389/fimmu.2019.01952 Leary, S., Underwood, W., Anthony, R., Cartner, S., Corey, D., Grandin, T., Greenacre, C., Gwaltney-Brant, S., McCrackin, M.A., Meyer, R., Miller, D., Shearer, J. and Yanong, R. 2013. AVMA guidelines for the euthanasia of animals: 2013 edition. Available via https://www.avma.org/KB/Policies/Documents/euthanasia.pdf Martınez, A.P., Delgado, P.J., Forriol, F. and Maffulli, N. 2011. Non-surgical therapies for peripheral nerve injury. Br. Med. Bull. 100, 73–100. Mathieu, L., Adam, C., Legagneux, J., Bruneval, P. and Masmejean, E. 2012. Reduction of neural scarring after peripheral nerve suture: an experimental study about collagen membrane and autologous vein wrapping. Chir. Main. 31(6), 311–317. Muir, P., Hans, E.C., Racette, M., Volstad, N., Sample, S.J., Heaton, C., Holzman, G., Schaefer, S.L., Bloom, D.D., Bleedorn, J.A., Hao, Z., Amene, E., Suresh, M. and Hematti, P. 2016. Autologous bone marrow-derived mesenchymal stem cells modulate molecular markers of inflammation in dogs with cruciate ligament rupture. PLoS One 11(8), e0159095; doi:10.1371/journal.pone.0159095 Nieto, R.L., Avila, P.L.M., Avila, G.J.P., Pérez, P. and Santamaria, E. 2018. Comparison of regenerative changes in peripheral nerve injuries treated with a regenerative tube with human mesenchymal cells and platelet-rich plasma lysate versus a nerve graft in rats. Int. J. Transplant. Plastic. Surg. 20(5), S28. Papakonstantinou, N.A., Baikoussis, N.G., Goudevenos, J., Papadopoulos, G. and Apostolakis, E. 2016. Novel no touch technique of saphenous vein harvesting: Is great graft patency rate provided? Ann. Card. Anesth. 19(3), 481–488. Pereira, T., Gärtner, A., Amorim, I., Almeida, A., Caseiro, A.R., Armada-da-Silva, P.A., Amado, S., Fregnan, F., Varejão, A.S., Santos, J.D., Bartolo, P.J., Geuna, S., Luís, A.L. and Mauricio, A.C. 2014. Promoting nerve regeneration in a neurotmesis rat model using poly(DL-lactide-epsilon-caprolactone) membranes and mesenchymal stem cells from the Wharton’s jelly: in vitro and in vivo analysis. Biomed. Res. Int. 2014, 302659; doi:10.1155/2014/302659 Ryu, J., Beimesch, C.F. and Lalli, T.J. 2011. Peripheral nerve repair. Orthop. Trauma. 25(3), 174–180. Sanchez, M., Garate, A., Delgado, D. and Padilla, S. 2017. Platelet-rich plasma, an adjuvant biological therapy to assist peripheral nerve repair. Neural Regen. Res. 12(1), 47–52. Sinis, N., Drakotos, A.K., Doser, M., Schlosshauer, B., Müller, E.S., Bruck, J.C. and Werdin, F. 2011. Bioartificial reconstruction of peripheral nerves using the rat median nerve model. Ann. Anat. 193(4), 341–346. Somasundaram, I., Mishra, R., Radhakrishnan, H., Sankaran, R., Garikipati, V.N.S. and Marappagounder, D. 2015. Human adult stem cells maintain a constant phenotype profile irrespective of their origin, basal media, and long term cultures. Stem Cell Int. 2015, 146051; doi:10.1155/2015/146051 Wang, P., Zhang, Y., Zhao, J. and Jiang, B. 2015. Intramuscular injection of bone marrow mesenchymal stem cells with small gap neurorrhaphy for peripheral nerve repair. Neurosci. Lett. 585, 119–125. Wasterlain, A.S., Braun, H.J. and Dragoo, J.L. 2012. Contents and formulations of platelet-rich plasma. Oper. Tech. Orthopaed. 22(1), 33–42. | ||
| How to Cite this Article |
| Pubmed Style Daradka MH, Ismail ZAB, Irsheid MA. Peripheral nerve regeneration: a comparative study of the effects of autologous bone marrow-derived mesenchymal stem cells, platelet-rich plasma and lateral saphenous vein graft as a conduit in a dog model. Open Vet. J.. 2021; 11(4): 686-694. doi:10.5455/OVJ.2021.v11.i4.20 Web Style Daradka MH, Ismail ZAB, Irsheid MA. Peripheral nerve regeneration: a comparative study of the effects of autologous bone marrow-derived mesenchymal stem cells, platelet-rich plasma and lateral saphenous vein graft as a conduit in a dog model. https://www.openveterinaryjournal.com/?mno=124350 [Access: January 25, 2026]. doi:10.5455/OVJ.2021.v11.i4.20 AMA (American Medical Association) Style Daradka MH, Ismail ZAB, Irsheid MA. Peripheral nerve regeneration: a comparative study of the effects of autologous bone marrow-derived mesenchymal stem cells, platelet-rich plasma and lateral saphenous vein graft as a conduit in a dog model. Open Vet. J.. 2021; 11(4): 686-694. doi:10.5455/OVJ.2021.v11.i4.20 Vancouver/ICMJE Style Daradka MH, Ismail ZAB, Irsheid MA. Peripheral nerve regeneration: a comparative study of the effects of autologous bone marrow-derived mesenchymal stem cells, platelet-rich plasma and lateral saphenous vein graft as a conduit in a dog model. Open Vet. J.. (2021), [cited January 25, 2026]; 11(4): 686-694. doi:10.5455/OVJ.2021.v11.i4.20 Harvard Style Daradka, M. H., Ismail, . Z. A. B. & Irsheid, . M. A. (2021) Peripheral nerve regeneration: a comparative study of the effects of autologous bone marrow-derived mesenchymal stem cells, platelet-rich plasma and lateral saphenous vein graft as a conduit in a dog model. Open Vet. J., 11 (4), 686-694. doi:10.5455/OVJ.2021.v11.i4.20 Turabian Style Daradka, Mousa H, Zuhair A Bani Ismail, and Mohammad A Irsheid. 2021. Peripheral nerve regeneration: a comparative study of the effects of autologous bone marrow-derived mesenchymal stem cells, platelet-rich plasma and lateral saphenous vein graft as a conduit in a dog model. Open Veterinary Journal, 11 (4), 686-694. doi:10.5455/OVJ.2021.v11.i4.20 Chicago Style Daradka, Mousa H, Zuhair A Bani Ismail, and Mohammad A Irsheid. "Peripheral nerve regeneration: a comparative study of the effects of autologous bone marrow-derived mesenchymal stem cells, platelet-rich plasma and lateral saphenous vein graft as a conduit in a dog model." Open Veterinary Journal 11 (2021), 686-694. doi:10.5455/OVJ.2021.v11.i4.20 MLA (The Modern Language Association) Style Daradka, Mousa H, Zuhair A Bani Ismail, and Mohammad A Irsheid. "Peripheral nerve regeneration: a comparative study of the effects of autologous bone marrow-derived mesenchymal stem cells, platelet-rich plasma and lateral saphenous vein graft as a conduit in a dog model." Open Veterinary Journal 11.4 (2021), 686-694. Print. doi:10.5455/OVJ.2021.v11.i4.20 APA (American Psychological Association) Style Daradka, M. H., Ismail, . Z. A. B. & Irsheid, . M. A. (2021) Peripheral nerve regeneration: a comparative study of the effects of autologous bone marrow-derived mesenchymal stem cells, platelet-rich plasma and lateral saphenous vein graft as a conduit in a dog model. Open Veterinary Journal, 11 (4), 686-694. doi:10.5455/OVJ.2021.v11.i4.20 |