| Original Article | ||
Open Vet. J.. 2021; 11(4): 755-763 Open Veterinary Journal, (2021), Vol. 11(4): 755–763 Original Research Quality of life assessment in cancer patients receiving single-agent versus multidrug chemotherapy protocolsMarco Luigi Bianchi1,2, Dario Drudi2, Elisabetta Treggiari3, Chiara Catalucci1, Valeria Attorri4, Irene Bonazzi4 and Paola Valenti1,4*1Clinica Veterinaria Malpensa, Samarate (VA), Italy 2Clinica Veterinaria Nervianese, Nerviano (MI), Italy 3Centro Specialistico Veterinario, Milano (MI), Italy 4Ospedale Veterinario i Portoni Rossi, Zola Predosa (BO), Italy *Corresponding Author: Paola Valenti. Clinica Veterinaria Malpensa, Samarate (VA), Italy. Email: pvalenti.dvm [at] gmail.com Submitted: 13/09/2021 Accepted: 11/11/2021 Published: 05/12/2021 © 2021 Open Veterinary Journal
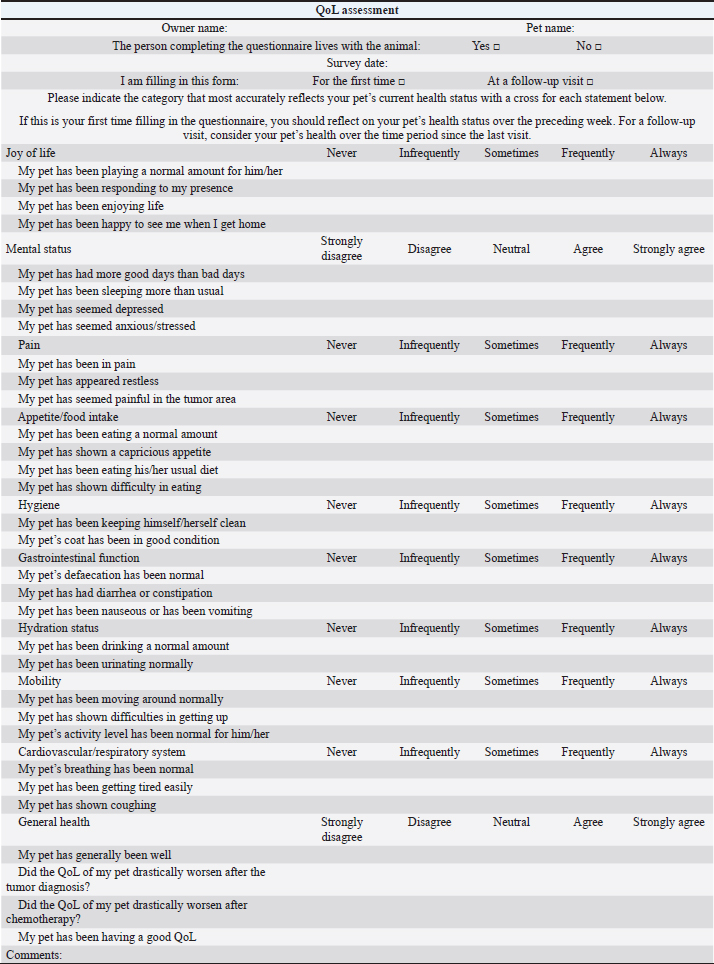
AbstractBackground: Quality of life (QoL) is an essential factor in therapeutic decision-making for human patients and is commonly used as an endpoint in clinical trials of cancer treatments. Aim: To compare owners’ perception of QoL in canine and feline patients affected by different tumor histotypes treated with single-agent or multidrug protocols. Methods: Owners were asked to assess the impact on QoL of their pets undergoing chemotherapy treatment by answering a questionnaire and assigning a score to different health-related parameters reported to affect QoL. Results: Questionnaires of 101 patients (85 dogs and 16 cats), collected at different time points, were analyzed. Fifty-seven patients were given single-agent chemotherapy (carboplatin, doxorubicin, lomustine, melphalan, mitoxantrone, vinblastine, and vinorelbine), whereas 44 were given multiple-agent treatment. When diverse factors including chemotherapy treatment type (single-agent vs. multidrug regimens) and the onset and kind of adverse effects were considered, no significant variations in owners’ perceptions of their pets’ QoL were discovered. Conclusion: Chemotherapy type (single-agent vs. multidrug protocol) and related adverse events are shown, which did not influence owners’ perception of their pet’s QoL. Future prospective studies should look into clinical characteristics that might affect QoL, such as the patient’s age, tumor stage, and protocol purpose (curative vs. palliative). Keywords: Canine, Chemotherapy, Feline, Quality of life, Tumor. IntroductionQuality of life (QoL) as a measurable parameter is a relatively recent concept, which has gained increasing importance in veterinary medicine over the last decade (Yeates and Main, 2009). There is no universally accepted definition of QoL, but it is generally considered a multidimensional concept that involves subjective evaluation of factors that contribute to the overall well-being (Osaba, 2011; Giuffrida and Kerrigan, 2014; Yousefi et al., 2016). One of the fundamental goals in managing cancer patients, especially in a palliative setting, is to maintain the best possible QoL, independently of the tumor type affecting the patients and despite any implemented treatment (Hamilton et al., 2012). As perceived by owners, poor QoL has been reported as a common reason for euthanasia (Edney, 1998), and therapeutic success is also defined based on owners’ perception of their pets’ QoL (Levine et al., 2008). In veterinary oncology, several studies have focused on the impact of a particular type of chemotherapy protocol on owners’ perception of their pets’ QoL. Bowles et al. (2010) showed that the majority of owners positively described the experience of their animals undergoing carboplatin-based chemotherapy. Two studies have so far evaluated owners’ perception of QoL in dogs and cats affected by lymphoma (LSA) and receiving multidrug protocols; in both cases, owners reported that the treatment did not negatively impact the QoL of their pet (Mellanby et al., 2003; Tzannes et al., 2008). When deciding on a course of antineoplastic treatment, most owners consider several factors, including prognosis, time commitment, costs, and potential occurrence of adverse events (AEs). When compared to single-agent protocols in human medicine, multidrug protocols may increase response rates and even prolong progression-free survival, despite being associated with an increased rate of treatment-associated AEs (Kumar and Chakraborty, 2016). However, two early studies showed that increased toxicity does not always negatively impact QoL (Funaioli et al., 2008; Huober and Thurlimann, 2009). In most cases, when exploring the impact of AEs on QoL in human medicine, not only gastrointestinal and hematological toxicity are evaluated, but also more specific chemotherapy-related AEs (such as hand–foot syndrome and peripheral neuropathy) and cosmetic side effects (such as alopecia), potentially affecting the patient’s perception of QoL (Funaioli et al., 2008). Single-agent protocols in veterinary medicine have historically and anecdotally been associated with fewer side effects than multidrug protocols, and thus may be offered by clinicians as a first-line treatment option in elderly patients or in cases where owners do not accept the risk of their pet experiencing severe AEs (Moore and Frimberger, 2018). In a previous study evaluating factors potentially influencing owners to treat their pet with chemotherapy, vomiting was considered an acceptable side effect, but inappetence, weight loss, and depression were deemed to be unacceptable (Williams et al., 2017). At present, no studies are assessing any differences in QoL, as perceived by pet owners, in dogs and cats receiving single-agent versus multidrug protocols, and there are no results to guide clinicians when discussing treatment options for pets with cancer. The authors’ primary goal was to assess owners’ perceptions of their pets’ QoL while they were receiving maximum tolerated dose (MTD) chemotherapy, either as a single agent or as part of a multidrug protocol; as a secondary goal, they wanted to identify clinical factors that could be linked to a perceived decrease in QoL. Materials and MethodsA modified, translated version of a previously published questionnaire (Lynch et al., 2010) (Table 1) was given to owners of dogs and cats with newly diagnosed different tumor types, receiving MTD chemotherapy as part of a single-agent or multidrug protocol. Questionnaires were collected from four different European institutions from 2018 until 2019. Owners were asked to fill the questionnaire at different time points during the chemotherapy course, specifically prior to treatment start, while receiving chemotherapy, and at the end of the protocol. All the drugs used were administered at their published MTD; single-agent chemotherapy was defined as a protocol including one cytotoxic drug, whereas multidrug protocols included different chemotherapy agents. Single-agent protocols included carboplatin (Rassnick et al., 2001; Kisseberth et al., 2008), chlorambucil (Vail et al., 2020), doxorubicin (Gustafson and Bailey, 2020), gemcitabine (Elpiner et al., 2011), lomustine (Gustafson and Bailey, 2020), melphalan (Fernandez and Chon, 2018), mitoxantrone (Lucroy et al., 1998), vinblastine (Bailey et al., 2008), and vinorelbine (Wouda et al., 2015), whilst multidrug protocols included carboplatin/doxorubicin (Bailey et al., 2003), carboplatin/5-fluorouracil (Menard et al., 2018), cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) (Vail et al., 2020), cyclophosphamide, vincristine, cytosine arabinoside, prednisone (COAP) (Hosoya et al., 2007), cyclophosphamide, vincristine, prednisone (COP) (Teske et al., 2002; Borgatti Jeffreys et al., 2005), dexamethasone, melphalan, actinomycin D, cytosine arabinoside (DMAC) (Alvarez et al., 2006), lomustine, vincristine, procarbazine, prednisone (LOPP) (Brown et al., 2018), vincristine, doxorubicin, cyclophosphamide (VAC) (Alvarez et al., 2013), and vinblastine/lomustine (Cooper et al., 2009). Treatment choice was clinician-dependent and based on their discussion with the owners, considering different factors, including tumor type, potential side effects, and costs. Signalment, tumor type, and disease stage were all documented, as well as protocol type, dosages, number of doses administered, and acute AEs. AEs were divided into three categories: gastrointestinal, hematological, and miscellaneous (hepatotoxicity, cardiotoxicity, and lethargy), and were rated using the Veterinary Co-operative Oncology Group (VCOG) scale (VCOG-CTCAE, 2016). Only those questionnaires that were filled in all their parts were included and used for the statistical analysis. Statistics was performed using a commercial software (Statistical Package for the Social Sciences 24, IBM Corp., New York, NY). QoL score was measured considering the questions: “Did the QoL of my pet drastically worsen after being diagnosed with cancer?” [Likert scale 1–5; 1=totally agree (worst QoL), 5=totally disagree (best QoL)] and “Did the QoL of my pet drastically worsen after starting the chemotherapy protocol?” (same scale). The scores for each question was assessed for normality using the Shapiro–Wilk and Kolmogorov–Smirnov tests, but they both rejected normality. Hence, using the Mann–Whitney U test, the median scores were compared for those groups identified by the possible significant variables: species (dog vs. cat), chemotherapy (single-agent vs. multidrug protocol), side effects (presence vs. absence; hematological vs. others; hematological vs. gastrointestinal; others vs. gastrointestinal), tumor type [mast cell tumor (MCT), LSA, osteosarcoma (OSA), other tumor types]. p-values were considered significant if <0.05. Ethical approvalAll procedures were carried out in accordance with institutional guidelines under the control of the Italian Ministry of Public Health (Italian Law D.lgs 26/2014). ResultsAnimalsA total of 101 patients met the inclusion criteria. Patients included 85 dogs and 16 cats; dogs were mostly crossbred (n=27, 31.8%), followed by Golden Retriever (n=7, 8.2%), and 4 of each of the following: English setter, French bulldog, and beagle (4.7%); the remainder was represented by other breeds (n=39, 45.9%). The median age was 9 years (range=3–14 years), gender included female dogs (n=47, 55%), of which 44 were neutered and 3 entire females, and male dogs (n=38, 45%), of which 14 were neutered and 24 entire males. Table 1. Questionnaire.
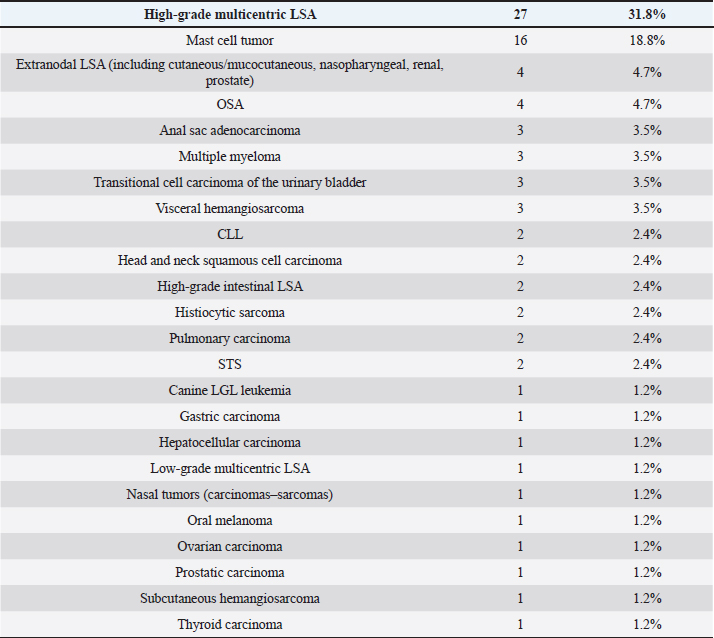
Table 2. Tumor types in dogs.
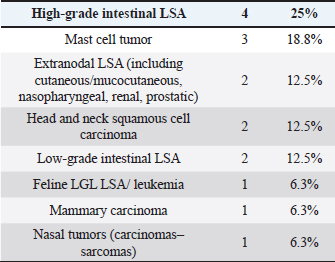
Cats were mostly domestic shorthair (n=14, 87.5%), followed by Ragdoll and Siamese (one each, 6.25%); median age was 12 years (range=6–15 years), eight cats were neutered females and eight neutered males. Tumor typesTwenty-seven tumor types were diagnosed (Tables 2 and 3). High-grade multicentric LSA was the most common in dogs (n=27, 31.8%), followed by cutaneous and subcutaneous MCT (n=16, 18.8%). Gastrointestinal LSA was the most common in cats (n=4, 25%), followed by cutaneous MCT (n=3, 18.7%). Chemotherapy protocolsWhen used as a single agent, chemotherapy included the following drugs: vinblastine (17), carboplatin (15), mitoxantrone (6), chlorambucil (5), lomustine (5), melphalan (5), vinorelbine (2), doxorubicin (1), and gemcitabine (1.) Multidrug protocols were as follows: CHOP (26), COP (7), LOPP (4), VAC (2), DMAC (1), COAP (1), carboplatin/doxorubicin (1), carboplatin/5-fluorouracil (1), and vinblastine/lomustine (1). Adverse eventsOf 101 patients, 51 (50.5%) did not show any AEs, while 50 animals (49.5%) showed at least one AEs. Twenty-three (39%) patients experienced gastrointestinal AEs (56% Grade 1, 39% Grade 2, and 5% Grade 4); 29 (49%) developed hematological toxicity, including 22 (76%) neutropenic events (41% Grade 1, 32% Grade 2, 18% Grade 3, and 9% Grade 4), 7 (24%) thrombocytopenic events (14% Grade 1, 44% Grade 2, 14% Grade 3, 14% Grade 4, and14% Grade 5); 2 dogs (3%) developed hepatotoxicity (Grade 2), 1 dog (2%) had cardiotoxicity (Grade 2), and 4 dogs (7%) developed lethargy (Grade 2). Table 3. Tumor types in cats.
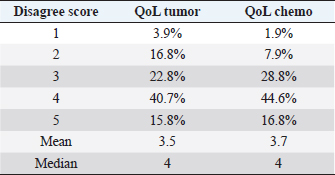
Eight patients experienced multiple side effects: five (10%) had both hematological and gastrointestinal AEs, one (2%) had gastrointestinal AEs and lethargy, one (2%) had hematological AEs and lethargy, and one (2%) had both gastrointestinal and hematological AEs and lethargy. When looking for adverse effects at any possible association between QoL and chemotherapy protocol (question: “Did the QoL of my pet drastically worsen after the chemotherapy protocol?”), analysis of the data showed no statistically significant difference between the two groups (single-agent vs. multidrug protocol, p=0.189). Questionnaire answersRegarding the QoL score measurement, 16 owners (15.8%) stated that they strongly disagreed about a decrease in QoL of their pet following diagnosis, 41 (40.7%) expressed disagreement, 23 (22.8%) were neutral, 17 (16.8%) agreed, and only 4 (3.9%) strongly agreed. Regarding a possible association between QoL and tumor diagnosis (question: Did the QoL of my pet drastically worsen after the diagnosis?), data analysis showed that there was no statistically significant difference between the two groups (single-agent vs. multidrug protocol, p=0.462). Seventeen owners (16.8%) stated that they strongly disagreed about a decrease in QoL due to chemotherapy treatment, 45 (44.6%) disagreed, 29 (28.8%) were neutral, 8 (7.9%) agreed, and only 2 (1.9%) strongly agreed (Table 4). Table 4. Questionnaire answers.
When looking at any possible association between QoL and chemotherapy protocol (question: “Did the QoL of my pet drastically worsen after the chemotherapy protocol?”), analysis of the data showed no statistically significant difference between the two groups (single-agent vs. multidrug protocol, p=0.189). Significant variablesThe perception of decreased QoL due to the presence of tumor was significantly different between dogs and cats (p=0.026), with a mean score of 2.88 for cat owners and 3.59 for dog owners. Therefore, QoL perception remained positive for dogs, while it strayed into negative for cats. When adjusting for tumor types, results showed that MCT was the least impacting QoL; the scores were relatively high, namely 4.11 for the first question and 4.16 for the second, showing significant differences (p=0.040 and 0.007, respectively) compared with other tumor types. Also, LSA and OSA did not significantly impact the QoL, while other tumor types showed a significant effect on QoL (p=0.040 and 0.043, respectively). Prostatic carcinoma, breast carcinoma, nasal tumors, chronic lymphocytic leukemia (CLL), large granular lymphocyte (LGL) leukemia, and high-grade intestinal LSA had the worst scores when assessing data linked to the question “Did the QoL of my pet drastically worsen after being diagnosed with cancer?” On the contrary, tumors with the best score were thyroid carcinoma, MCT, soft tissue sarcoma (STS), and subcutaneous hemangiosarcoma (HSA). When it came to the second question, “Did my pet’s QoL drastically worsen after starting the chemotherapy protocol?” Dogs treated for pulmonary carcinoma, LGL leukemia, and multicentric LSA had the best QoL score, while dogs treated for MCT, STS, thyroid cancer, subcutaneous HSA, and mammary carcinoma had the worst scores. To conclude, there were no significant variations in owners’ perceptions of QoL based on the presence or absence of AEs, the type of AEs generated, or the type of chemotherapy (single-agent vs. multidrug regimen) (p > 0.05). DiscussionIn the last 40 years, we have been faced with a change in the tasks of veterinary medicine and QoL assessment has now become an important component of veterinary oncology, both in clinical research and during the daily clinical assessment (Belshaw et al., 2015), with an exponential increase in the number of published articles about this topic (Mellanby et al., 2003; Tzannes et al., 2008; Lynch et al., 2010; Vøls et al., 2017). In human medicine, protocol’s choice is mainly based on reported treatment response, expected toxicity, patient preference, disease stage (e.g., advanced disease or presence of metastases for solid tumors), or imminent complications requiring aggressive and rapid tumor control (Grünberger et al., 2007; Kumar and Chakraborty, 2016; Petrelli et al., 2020). Several studies in human medicine have shown that multidrug versus single-agent protocols are associated with improved overall survival and response rate without necessarily worsening QoL (Funaioli et al., 2008; Yalcin et al., 2020), which appears to be unrelated to the toxicity profile of the specific protocol (Funaioli et al., 2008). Also, in veterinary medicine, especially when analyzing the available literature on multicentric LSA treatment, multidrug compared to single-agents protocols have been associated with increased response rate and overall survival (Valerius et al., 1997; Al Nadaf et al., 2018); nevertheless, we tend to choose the type of chemotherapy based on several factors, including tumor type, type and interval of administrations, expected AEs, owners’ acceptance of potential side effects and costs. The major goal of our study was to determine how owners felt about their dogs’ QoL while they were receiving an MTD protocol, whether it was a single-agent or a multidrug protocol, and to uncover clinical characteristics that may be linked to a drop in QoL. No significant differences were found when considering the presence or absence of AEs, type of developed AEs, and the chemotherapy protocol used (single-agent vs. multidrug protocols). Therefore, based on these data, the chemotherapy protocol of choice should be merely based on medical considerations, rather than concerns regarding the consequences of owners’ perception of QoL. Half of the patients (51%) in both groups did not show any toxicity when considering side effects. All the VCOG toxicity grades, ranging from 1 to 4, were included in the study but, due to low numbers, types, and grades of AEs in different groups (e.g., hematological, gastrointestinal, others), could not be statistically compared and associated to their impact on QoL. A correlation has been reported in human medicine where the severity of chemotherapy-related AEs, mainly classified as grade 3 or above, were strongly correlated with the QoL of patients with advanced cancer (Park et al., 2016). Regarding the species, the perception of decreased QoL following the cancer diagnosis was significantly different between dogs and cats, and QoL perception remained positive for dogs while strayed into negative for cats. The varied habits and behavior of dogs versus cats, as well as the varying time spent by the owner in the indoor and outside environment with their pet, might be a plausible reason for this outcome. When looking at specific tumor types, our study found that LSA did not seem to affect QoL: this is in agreement with previously reported results in both dogs and cats, where the QoL of LSA patients was not affected (Mellanby et al., 2003; Tzannes et al., 2008; Thornton et al., 2018). MCT was the tumor to be least impacting QoL; this is not surprising since most patients included in the study received adjuvant chemotherapy with vinblastine to treat microscopic disease. Furthermore, OSA had no negative impact on QoL; one possible explanation for this finding is that most OSA patients had already undergone limb amputation and were thus pain-free when chemotherapy treatment began; however, due to the small number of cases, we are unable to draw any meaningful conclusions from this data. Prostatic carcinoma, breast carcinoma, nasal tumors, CLL, LGL leukemia, and high-grade intestinal LSA were among the tumors with the worst scores, which is not unexpected given that chemotherapy was used as a palliative treatment or as the sole therapeutic option. In the present study, a wide number of tumor types was included: again, as a consequence of the lack of numerosity in the single groups, data concerning the correlation between tumor type and QoL should be interpreted with caution. This study has several limitations, including the absence of a validated method to assess QoL in veterinary oncology patients. Many reviews have focused on the validation of different methods used to measure QoL (McMillan, 2000; Wojciechowska and Hewson, 2005; Giuffrida and Kerrigan, 2014; Belshaw et al., 2015; Vøls et al., 2017) and, even if recently Giuffrida et al. (2018) proposed a psychometric test to standardize the measurement of QoL, tools to measure QoL in pets suffering from cancer need to be validated in the future. One of the significant differences between human and veterinary medicine in defining patients’ QoL is that, in human medicine, this parameter is, in most cases, stated by the patient himself. On the contrary, veterinary medicine is determined by the owner or, alternatively, by the clinician or both (McMillan, 2000). Additional QoL evaluation domains in addition to the classic clinical and physical parameters have been added to our questionnaire, as previously reported by Vøls et al. (2017), and should be routinely considered in the assessment of QoL in veterinary patients, based on models such as the PedsQLTM scale designed to assess QoL in children (http://www.pedsql.org.). The low number of patients represents another limitation of the study included in each subgroup and the heterogeneity of our groups in terms of tumor types and chemotherapy protocols used; for this reason, patients could not be stratified based on the type or combination of used drugs and the degree of recorded side effects; also, a potential association between tumor characteristics (tumor type, disease stage) and owner’s perception of QoL could not be investigated further. Also, the questionnaires were not anonymous, and it is possible that anonymous answers could have increased the chance of retrieving scores with a more negative trend. Finally, this study included only a referral population, thus creating a bias regarding the type of cases and owners that could have shown a higher motivation and were possibly more willing to accept and tolerate chemotherapy AEs. To conclude, our study suggests that type of chemotherapy protocol and related AEs did not affect owners’ awareness of their pet QoL. Therefore, a multidrug protocol appears to be well tolerated by the owners of pets undergoing chemotherapy. It should be discussed and offered more often, independently of the perceived influence of potential AEs on QoL. Future prospective studies looking for clinical factors possibly related to QoL, such as species, age, tumors histotypes, stage of the disease, and intent of the protocol (curative vs. palliative), are warranted. Conflict of interestNone of the authors has any other financial or personal relationships that could inappropriately influence or bias the paper’s content. Authors’ contributionMLB and PV conceived and supervised the study; DD performed statistical analysis; ET supervised the study, was directly responsible for the clinical cases, and assisted during data collection. CC, VA, and IB were directly responsible for the clinical cases and assisted during data collection. ReferencesAl Nadaf, S., Rebhun, R.B., Curran, K.M., Venable, R.O., Skorupski, K.A., Willcox, J.L. and Burton, J.H. 2018. Retrospective analysis of doxorubicin and prednisone as first-line therapy for canine B-cell lymphoma. BMC Vet. Res. 14(356), 1–8. Alvarez, F.J., Kisseberth, W.C., Gallant, S.L. and Couto, C.G. 2006. Dexamethasone, melphalan, actinomycin D, cytosine arabinoside (DMAC) protocol for dogs with relapsed lymphoma. J. Vet. Intern. Med. 20, 1178–1183. Alvarez, F.J., Hosoya, K., Lara-Garcia, A., Kisseberth, W. and Couto, G. 2013. VAC protocol for treat- ment of dogs with stage III hemangiosarcoma. J. Am. Anim. Hosp. Assoc. 49, 370–377. Bailey, D., Erb, H., Williams, L., Ruslander, D. and Hauck, M. 2003. Carboplatin and doxorubicin combination chemotherapy for the treatment of appendicular osteosarcoma in the dog. J. Vet. Intern. Med. 17, 199–205. Bailey, D.B., Rassnick, K.M., Kristal, O., Chretin, J.D. and Balkman, C.E. 2008. Phase I dose escalation of single-agent vinblastine in dogs. J. Vet. Intern. Med. 22, 1397–1402. Belshaw, Z., Asher, L., Harvey, N.D. and Dean, R.S. 2015. Quality of life assessment in domestic dogs: an evidence-based rapid review. Vet. J. 206(2), 203–212. Borgatti Jeffreys, A., Knapp, D.W., Carlton, W.W., Thomas, R.M., Bonney, P.L., deGortari, A. and Lucroy, M.D. 2005. Influence of asparaginase on a combination chemotherapy protocol for canine multicentric lymphoma. J. Am. Anim. Hosp. Assoc. 41, 221–226. Bowles, D.B., Robson, M.C., Galloway, P.E. and Walker, L. 2010. Owners’ perception of carboplatin in conjunction with other palliative treatments for cancer therapy. J. Small Anim. Pract. 51(2), 104–112. Brown, P.M., Tzannes, S., Nguyen, S., White, J. and Langova, V. 2018. Lopp chemotherapy as a first-line treatment for dogs with T-cell lymphoma. Vet. Comp. Oncol. 16, 108–113. Cooper, M., Tsai, X. and Bennett, P. 2009. Combination CCNU and vinblastine chemotherapy for canine mast cell tumours: 57 cases. Vet. Comp. Oncol. 7, 196–206. Edney, A.T. 1998. Reasons for the euthanasia of dogs and cats. Vet. Rec. 143(4), 114. Elpiner, A.K., Brodsky, E.M., Hazzah, T.N. and Post, G.S. 2011. Single-agent gemcitabine chemotherapy in dogs with hepatocellular carcinomas. Vet. Comp. Oncol. 9, 260–268. Fernandez, R. and Chon, E. 2018. Comparison of two melphalan protocols and evaluation of outcome and prognostic factors in multiple myeloma in dogs. J. Vet. Intern. Med. 32, 1060–1069. Funaioli, C., Longobardi, C. and Martoni, A.A. 2008. The impact of chemotherapy on overall survival and quality of life of patients with metastatic colorectal cancer: a review of phase III trials. J. Chemother. 20(1), 14–27. Giuffrida, M.A. and Kerrigan, S.M. 2014. Quality of life measurement in prospective studies of cancers treatments in dogs and cats. J. Vet. Intern. Med. 28(6), 1824–1829. Giuffrida, M.A., Brown, D.C., Ellenberg, S.S. and Farrar, J.T. 2018. Development and psychometric testing of the canine owner-reported quality of life questionnaire, an instrument designed to measure quality of life in dogs with cancer. J. Am. Vet. Med. Assoc. 252(9), 1073–1083. Grünberger, B., Raderer, M., Schmidinger, M. and Hejna M. 2007. Palliative chemotherapy for recurrent and metastatic esophageal cancer. Anticancer Res. 27(4C), 2705–2714. Gustafson, D.L. and Bailey, D.B. 2020. Cancer chemotherapy. In Small animal clinical oncology. Eds., Vail, D.M., Thamm, D.H. and Liptak, J.M. Elsevier, St. Louis, Missouri, pp: 182–208. Hamilton, M.J., Sarcornrattana, O., Iliopoulou, M., Xie Y. and Kitchell B. 2012. Questionnaire-based assessment of owner concerns and doctor responsiveness: 107 canine chemotherapy patients. J. Small Anim. Pract. 53(11), 627–633. Hosoya, K., Kisseberth, W.C., Lord, L.K., Alvarez, F.J., Lara-Garcia, A., Kosarek, C.E., London, C.A. and Couto, C.G. 2007. Comparison of COAP and UW-19 protocols for dogs with multicentric lymphoma. J. Vet. Intern. Med. 21, 1355–1363. Huober, J. and Thurlimann, B. 2009. The role of combination chemotherapy in the treatment of patients with metastatic breast cancer. Breast Care (Basel) 4(6), 367–372. Kisseberth, W.C., Vail, D.M., Yaissle, J., Jeglum, K.A., Couto, C.G., Ward, H., Khanna, C. and Obradovich, J.E. 2008. Phase I clinical evaluation of carboplatin in tumor-bearing cats: a Veterinary Cooperative Oncology Group study. J. Vet. Intern. Med. 22(1), 83–88. Kumar, A. and Chakraborty, B.S. 2016. A meta-analysis study of polychemotherapy versus monochemotherapy in patients of metastatic breast cancer, Int. J. Pharm. Sci. Drug Res. 8(03), 134–143. Levine, J.M., Budke, C.M., Levine, G.J., Kerwin, S.C., Hettlich, B.F. and Slater, M.R. 2008. Owner-perceived, weighted quality-of-life assessments in dogs with spinal cord injuries. J. Am. Vet. Med. Assoc. 233(6), 931–935. Lucroy, M.D., Phillips, B.S., Kraegel, S.A., Simonson, E.R. and Madewell, B.R. 1998. Evaluation of single- agent mitoxantrone as chemotherapy for relapsing canine lymphoma. J. Vet. Intern. Med. 12, 325–329. Lynch, S., Savary-Bataille, K., Leeuw, B. and Argyle, D.J. 2010. Development of a questionnaire assessing health-related quality-of-life in dogs and cats with cancer. Vet. Comp. Oncol. 9(3), 172–182. McMillan, F.D. 2000. Quality of life in animals. J. Am. Vet. Med. Assoc. 216(12), 1904–1910. Mellanby, R.J., Herrtage, M.E. and Dobson, J.M. 2003. Owners’ assessments of their dog’s quality of life during palliative chemotherapy for lymphoma. J. Small Anim. Pract. 44(3), 100–103. Menard, K., Flesner, B.K., Glahn, A., Boudreaux, B. and Bryan, J.N. 2018. Concurrent 5-fluorouracil and carboplatin for the treatment of canine carcinomas. Vet. Comp. Oncol. 16(4), 590–595. Moore, A.S. and Frimberger, A.E. 2018. Usefulness of chemotherapy for the treatment of very elderly dogs with multicentric lymphoma. J. Am. Vet. Med. Assoc. 252(7), 852–859. Osaba, D. 2011. Health-related quality of life and cancer clinical trial. Ther. Adv. Med. Oncol. 3(2), 57–71. Park, S.A., Hyun Chung, S. and Lee, L. 2016. Factors influencing the quality of life of patients with advanced cancer. Appl. Nurs. Res. 33, 108–112. Petrelli, F., Perego, G., Ghidini, A., Borgonovo, K., Scolari, C., Nozza, R., Rampulla, V., Costanzo, A., Varricchio, A., Rausa, E., Pietrantonio, F. and Zaniboni, A. 2020. A systematic review of salvage therapies in refractory metastatic colorectal cancer. Int. J. Colorectal Dis. 35(5), 783–794 Rassnick, K.M., Ruslander, D.M., Cotter, S.M., Al-Sarraf, R., Bruyette, D.S., Gamblin, R.M., Meleo, K.A. and Moore, A.S. 2001. Use of carboplatin for treatment of dogs with malignant melanoma: 27 cases (1989–2000). J. Am. Vet. Med. Assoc. 218(9), 1444–1448. Teske, E., van Straten, G., van Noort, R. and Ruttemann, G.R. 2002. Chemotherapy with cyclophosphamide, vincristine, and prednisolone (COP) in cats with malignant lymphoma: new results with an old protocol. J. Vet. Intern. Med. 16, 179–186. Thornton, L.A., Cave, N., Bridges, J.P. and Stell, A.J. 2018. Owner perceptions of their cat’s quality of life when treated with a modified University of Wisconsin-Madison protocol for lymphoma. J. Feline Med. Surg. 20(4), 356–361. Tzannes, S., Hammond, M.F., Murphy, S., Sparkes A. and Blackwood L. 2008. Owners’ perception of their cats’ quality of life during COP chemotherapy for lymphoma. J. Feline Med. Surg. 10(1), 73–81. Vail, D.M., Pinkerton, M. and Young, K.M. 2020. Hematopoietic tumors. In Small animal clinical oncology. Eds., Vail, D.M., Thamm, D.H. and Liptak, J.M. Elsevier, St. Louis, Missouri, pp: 688–772. Valerius, K.D., Ogilvie, G.K., Mallinckrodt, C.H. and Getzy, D.M. 1997. Doxorubicin alone or in combination with asparaginase, followed by cyclophosphamide, vincristine, and prednisone for treatment of multicentric lymphoma in dogs: 121 cases (1987–1995). J. Am. Vet. Med. Assoc. 210(4), 512–516. VCOG-CTCAE (Veterinary Cooperative Oncology Group—Common terminology criteria for adverse events). 2016. Following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. 2016. Vet. Comp. Oncol. 14(4), 417–446. Vøls, K.K., Heden, M.A., Kristensen, A.T. and Sandøe P. 2017. Quality of life assessment in dogs and cats receiving chemotherapy—a review of current methods. Vet. Comp. Oncol. 15(3), 684–691. Williams, J., Phillips, C. and Byrd, H.M. 2017. Factors which influence owners when deciding to use chemotherapy in terminally ill pets. Animals 7, 18. Wojciechowska, J.I. and Hewson, C.J. 2005. Quality-of-life assessment in pet dogs. J. Am. Vet. Med. Assoc. 226(5), 722–728. Wouda, R.M., Miller, M.E., Chon, E. and Stein, T.J. 2015. Clinical effects of vinorelbine administration in the management of various malignant tumor types in dogs: 58 cases (1997–2012). J. Am. Vet. Med. Assoc. 246, 1230–1237. Yalcin, S., Dane, F., Oksuzoglu, B., Ozdemir, N.Y., Isikdogan, A., Ozkan, M., Demirag, G.G., Coskun, H.S., Karabulut, B., Evrensel, T., Ustaoglu, M.A., Ozdemir, F., Turna, H., Yavuzsen, T., Aykan, F., Sevinc, A., Akbulut, H., Yuce, D., Hayran, M. and Kilickap, S. 2020. Quality of life study of patients with unresectable locally advanced or metastatic pancreatic adenocarcinoma treated with gemcitabine+nab-paclitaxel versus gemcitabine alone: AX-PANC-SY001, a randomized phase-2 study. BMC Cancer 20(1), 259. Yeates, J. and Main, D. 2009. Assessment of companion animal quality of life in veterinary practice and research. J. Small Anim. Pract. 50(6), 274–281. Yousefi, P., Rasekhi, S. and Heshmati, H. 2016. Quality of life in medical sciences. Int. J. Med. Res. Health Sci. 5, 43–46. | ||
| How to Cite this Article |
| Pubmed Style Bianchi ML, Drudi D, Treggiari E, Catalucci C, Attorri V, Bonazzi I, Valenti P. Quality of life assessment in cancer patients receiving single-agent vs. multidrug chemotherapy protocols. Open Vet. J.. 2021; 11(4): 755-763. doi:10.5455/OVJ.2021.v11.i4.28 Web Style Bianchi ML, Drudi D, Treggiari E, Catalucci C, Attorri V, Bonazzi I, Valenti P. Quality of life assessment in cancer patients receiving single-agent vs. multidrug chemotherapy protocols. https://www.openveterinaryjournal.com/?mno=133246 [Access: January 25, 2026]. doi:10.5455/OVJ.2021.v11.i4.28 AMA (American Medical Association) Style Bianchi ML, Drudi D, Treggiari E, Catalucci C, Attorri V, Bonazzi I, Valenti P. Quality of life assessment in cancer patients receiving single-agent vs. multidrug chemotherapy protocols. Open Vet. J.. 2021; 11(4): 755-763. doi:10.5455/OVJ.2021.v11.i4.28 Vancouver/ICMJE Style Bianchi ML, Drudi D, Treggiari E, Catalucci C, Attorri V, Bonazzi I, Valenti P. Quality of life assessment in cancer patients receiving single-agent vs. multidrug chemotherapy protocols. Open Vet. J.. (2021), [cited January 25, 2026]; 11(4): 755-763. doi:10.5455/OVJ.2021.v11.i4.28 Harvard Style Bianchi, M. L., Drudi, . D., Treggiari, . E., Catalucci, . C., Attorri, . V., Bonazzi, . I. & Valenti, . P. (2021) Quality of life assessment in cancer patients receiving single-agent vs. multidrug chemotherapy protocols. Open Vet. J., 11 (4), 755-763. doi:10.5455/OVJ.2021.v11.i4.28 Turabian Style Bianchi, Marco Luigi, Dario Drudi, Elisabetta Treggiari, Chiara Catalucci, Valeria Attorri, Irene Bonazzi, and Paola Valenti. 2021. Quality of life assessment in cancer patients receiving single-agent vs. multidrug chemotherapy protocols. Open Veterinary Journal, 11 (4), 755-763. doi:10.5455/OVJ.2021.v11.i4.28 Chicago Style Bianchi, Marco Luigi, Dario Drudi, Elisabetta Treggiari, Chiara Catalucci, Valeria Attorri, Irene Bonazzi, and Paola Valenti. "Quality of life assessment in cancer patients receiving single-agent vs. multidrug chemotherapy protocols." Open Veterinary Journal 11 (2021), 755-763. doi:10.5455/OVJ.2021.v11.i4.28 MLA (The Modern Language Association) Style Bianchi, Marco Luigi, Dario Drudi, Elisabetta Treggiari, Chiara Catalucci, Valeria Attorri, Irene Bonazzi, and Paola Valenti. "Quality of life assessment in cancer patients receiving single-agent vs. multidrug chemotherapy protocols." Open Veterinary Journal 11.4 (2021), 755-763. Print. doi:10.5455/OVJ.2021.v11.i4.28 APA (American Psychological Association) Style Bianchi, M. L., Drudi, . D., Treggiari, . E., Catalucci, . C., Attorri, . V., Bonazzi, . I. & Valenti, . P. (2021) Quality of life assessment in cancer patients receiving single-agent vs. multidrug chemotherapy protocols. Open Veterinary Journal, 11 (4), 755-763. doi:10.5455/OVJ.2021.v11.i4.28 |