| Case Report | ||
Open Vet. J.. 2024; 14(10): 2714-2720 Open Veterinary Journal, (2024), Vol. 14(10): 2714–2720 Case Report A case of primary pulmonary paraganglioma in a dogYoshimichi Goda1, Shinya Mizutani1,2*, Natsuki Akashi1,2, Teppei Kanda1,2, Kenji Kutara1,2, Yasuhiko Okamura1,2 and Taketoshi Asanuma1,21Okayama University of Science, Veterinary Medical Teaching Hospital, Imabari, Japan 2Faculty of Veterinary Medicine, Okayama University of Science, Imabari, Japan *Corresponding Author: Shinya Mizutani. Okayama University of Science, Veterinary Medical Teaching Hospital, Imabari, Japan. Email: s-mizutani [at] ous.ac.jp Submitted: 12/06/2024 Accepted: 03/09/2024 Published: 31/10/2024 © 2024 Open Veterinary Journal
AbstractBackground: Lung tumors in dogs, significantly primary paragangliomas, are rare and have not been reported. This report describes a dog with a lung tumor diagnosed as a primary paraganglioma. Case Description: A 12-year-old spayed French bulldog presented with a left-sided pulmonary mass. The dog was in good general condition and had no clinical symptoms. Computed tomography (CT) revealed a pulmonary mass near the bifurcation of the posterior lobe bronchus of the left lung. The mass showed a strong contrast enhancement effect that was subsequently attenuated. The dog underwent Surgery to remove the mass from the left lung. Abnormal hypertension was observed during surgery, and hypertensive crisis was suspected. Based on the histopathology and preoperative and postoperative urinary metanephrine and normetanephrine levels, the dog was diagnosed with primary paraganglioma of the lung. Although the CT scan showed findings suggestive of the development of a neuroendocrine tumor, it was difficult to suspect the development of a paraganglioma. Conclusion: The possibility of catecholamine-producing tumors should be considered when we encounter a lung tumor with no clinical symptoms and a neuroendocrine tumor-like contrast enhancement pattern on a CT scan. Keywords: Canine, Dog, Paraganglioma, Pulmonary. IntroductionThe incidence of pulmonary tumors in dogs is rare, less than 1% (Brodey and Craig, 1965; Moulton et al., 1981). Carcinoma is the most common occurrence (87.1%) (McPhetridge et al., 2021), and rare neuroendocrine tumors have also been reported (Saegusa et al., 1994: McPhetridge et al., 2021). The neuroendocrine tumors of the lung in dogs are few, and clinical information is limited. Canine neuroendocrine tumors generally include catecholamine-producing tumors, the most common of which are pheochromocytomas of the adrenal glands (Barthez et al., 1997). Extra-adrenal catecholamine-producing tumors are known as paragangliomas (Lunn and Boston, 2020), and there have been few reports on paragangliomas in dogs (Ilha and Styer, 2013; Rodrigues et al., 2020; Park and Minamoto, 2021; Hu et al., 2022). Although there have been reports of pulmonary metastases from pheochromocytomas, there have been no reports on the diagnosis of primary paragangliomas of the lungs (Barthez et al., 1997). In dogs, paragangliomas originating from the tongue, heart, retroperitoneum, and urinary bladder have been reported (Ilha and Styer, 2013; Rodrigues et al., 2020; Park and Minamoto, 2021; Hu et al., 2022). Previous paragangliomas in dogs have been reported to cause clinical symptoms such as anorexia and lethargy (Hu et al., 2022), but the clinical symptoms vary depending on the location of the disease (Ilha and Styer, 2013; Rodrigues et al., 2020; Wilson and Soto-Blanco, 2020; Park and Minamoto, 2021; Hu et al., 2022). Surgical excision is the preferred treatment (Yanagawa et al., 2014; Park and Minamoto, 2021; Hu et al., 2022; Tamura et al., 2023). To the best of the authors' knowledge, the maximum survival time for paragangliomas was reported to be 670 days (Robat et al., 2016; Rodrigues et al., 2020), while lung neuroendocrine tumors were reported to be 11 months (Choi et al., 2008). This report describes a dog with an incidentally found lung tumor that was diagnosed as a primary paraganglioma. Case DetailsA 12-year-old spayed female French bulldog weighing 12.2 kg was visited by a veterinarian for a medical checkup. The dog had no clinical symptoms and was in good general condition; however, chest radiography revealed a mass in the left thoracic region. The dog was referred to the Okayama University of Science Veterinary Medical Teaching Hospital for close examination and treatments (day 1). The dog’s temperature was 38.3°C, heart rate was 100 beats/minute, and respiratory rate was 40 beats/minute. The non-obstructive blood pressure was as follows: systolic arterial blood pressure (SAP), 168 mmHg; mean arterial blood pressure (MAP), 124 mmHg; and diastolic arterial blood pressure (DAP), 119 mmHg. No abnormal values were observed in complete blood counts. Blood chemistry tests showed mild hypercholesterolemia (474 mg/dl), mild elevation of alkaline phosphatase (ALP; 629 U/l), and alanine aminotransferase (86 U/l) levels, and a mild decrease in blood urea nitrogen (7.5 mg/dl). The dog presented with polyurea or polydipsia. In the ACTH stimulation test, the postcortisol level was 27.8 µg/dl. Therefore, it was determined that the case presented with Cushing's syndrome as an underlying disease. Computed tomography (CT) scan (Aquilion Lightning; Canon Medical Systems Co., Otawara, Japan) performed in general anesthesia. Iopamidol (Oypalomin 300, Fuji Pharma, Japan) was used as a contrast medium [injection volume; 2.5 ml/kg (750 mgI/kg), injection time; 15 seconds]. Precontrast (before the injection of contrast medium), arterial phase (15 seconds after the start of injection), venous phase (40 seconds after the start of injection), the equilibrium phase (120 seconds after the start of injection) scans were obtained. The mass (42 × 39 × 44 mm) showed in the basilar left caudal lobe. The mass was in contact with the left cranial lobe, was strongly contrast-enhanced in the venous phase, and rapidly washed out in the equilibrium phase (Fig. 1). The right middle tracheobronchial lymph nodes were enlarged and showed a contrast-enhancement pattern similar to that of the mass in the left caudal lobe (Fig. 2). An incidentally enlarged right adrenal gland is also observed. The right adrenal gland was enlarged to 10 mm, spherical with a smooth limbus, contrast-enhanced at the limbus, peaked in the venous phase, and was attenuated in the equilibrium phase (Fig. 3). Based on the CT findings, we tentatively diagnosed the patient with a primary lung neuroendocrine tumor with lymph node metastasis. The anesthesia was stable during the CT scan, and no significant blood pressure fluctuations were observed.
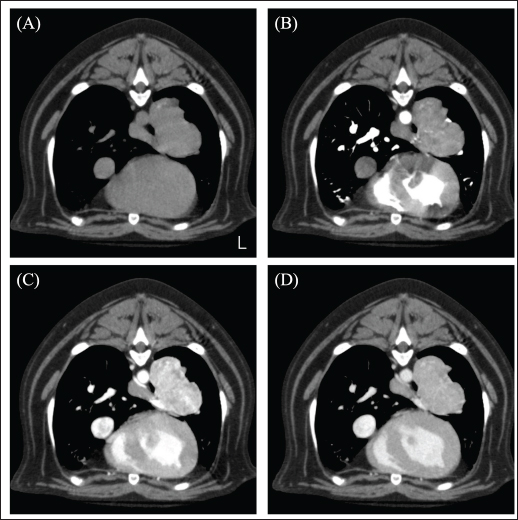
Fig. 1. CT image of the chest. (A) Plain, (B) arterial phase, (C) venous phase, and (D) equilibrium phase. The mass was in contact with the left cranial lobe, was strongly contrast-enhanced in the venous phase, and rapidly washed out in the equilibrium phase.
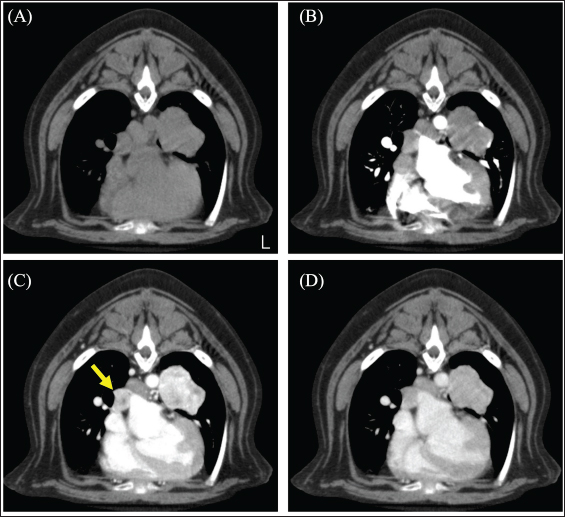
Fig. 2. CT image of the chest. (A) Plain, (B) arterial phase, (C) venous phase, and (D) equilibrium phase. The yellow arrow indicates the right middle tracheobronchial lymph node. The lymph nodes were strongly contrast-enhanced in the venous phase and rapidly washed out in the equilibrium phase. The mean CT values of lung tumors were 72.01 HU in the arterial phase, 206.57 HU in the venous phase, and 132.17 HU in the equilibrium phase. On day 51, the dog underwent a left caudal lobectomy of the lung. The subject was premedicated with atropine (40 μg/kg) injected subcutaneously and fentanyl (3 μg /kg) and ketamine (0.5 mg/kg) intravenously (IV). General anesthesia was induced with intravenous propofol and maintained by propofol-based total intravenous anesthesia accompanied by fentanyl and ketamine infusions. The nerve stimulation-guided paravertebral block was performed at the fifth to seventh intercostal space of the left thoracic wall with 0.5% bupivacaine. A right dorsal pedal artery was percutaneously cannulated with a 24-gage catheter for invasive arterial pressure measurement. An indwelling urinary catheter was placed to measure intraoperative urine volume. The surgery was performed by opening the left fifth intercostal space. After opening the chest, the left caudal lobe mass was identified, and the vascular system was processed in the following order: the pulmonary artery, lobar bronchus of the left cranial lung lobe, and pulmonary vein. The pulmonary arteries and lobar bronchioles were examined without any problems during processing. Sudden severe hypertension (SAP, 275 mmHg; MAP, 212 mmHg; DAP, 181 mmHg) occurred during surgical manipulation for vascularization of the pulmonary veins. Suspecting the onset of a hypertensive crisis, the dog was treated with a calcium channel blocker nicardipine (Sawai Pharmaceutical Co., Ltd., Osaka, Japan) 10 μg/kg IV for antihypertensive treatment; this improved the blood pressure to SAP 132 mmHg, MAP 94 mmHg, and DAP 75 mmHg and then the surgery was continued. For the purpose of yielding hemodynamic stability, a concomitant infusion of medetomidine and nicardipine at the rate of 1 and 5 μg/kg/hr, respectively, was initiated. Nevertheless, the antihypertensive rescue with nicardipine IV was repeated, thereafter, required because of repeatedly occurred hypertension (SAP, >200 mmHg) during the procedure of the mass. As the vascular processing of the mass progressed, the hypertension resolved, and the blood pressure stabilized (SAP 111 mmHg; MAP 74 mmHg; DAP 58 mmHg).
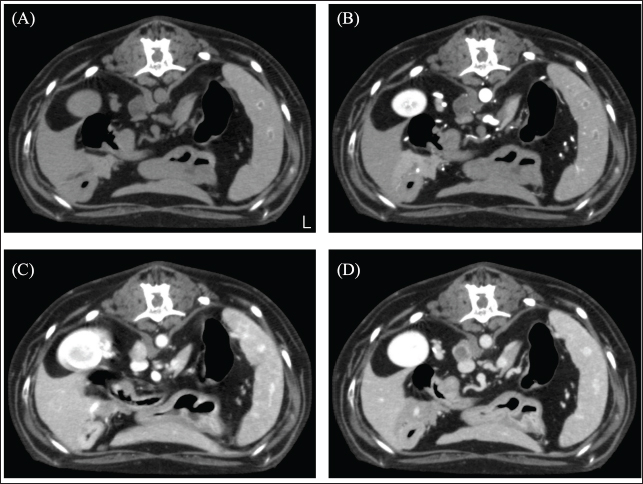
Fig. 3. CT image of the abdomen. (A) Plain, (B) arterial phase, (C) venous phase, and (D) equilibrium phase. The right adrenal gland was spherical with a smooth limbus, contrast-enhanced at the limbus, peaked in the venous phase, and attenuated in the equilibrium phase. The mean CT values of adrenal tumors were 22.23 HU in arterial phase, 87.58 HU in venous phase, and 48.21 HU in equilibrium phase. There was no obvious bleeding or air leakage at the resection surface. The chest was closed, and the surgery was terminated. The dog was in good postoperative condition and was discharged in the post-operative 3 day. Histopathological examination revealed cuboidal to polygonal cells densely arranged in a cord-like or band-like pattern. Proliferating cells had round-to-oval nuclei and moderate cytoplasmic, acidophilic, and basophilic granularity. Tumor cell infiltration was observed in the blood vessels. Immunohistochemical staining showed that the mass was strongly positive for the neuroendocrine markers Synaptophysin and Chromogranin A, diagnosed as a neuroendocrine carcinoma (Fig. 4). The dog had a hypertensive crisis intraoperatively; therefore, indwelling urinary metanephrine (MN) and normetanephrine (NMN) levels (Hokkaido University One Health Research Center; Hokkaido, Japan) were measured utilizing urine sample collected preoperatively from a urinary catheter. The preoperative urinary MN/creatinine (Cre) ratio of 904 (reference standard range: 0–120) and the NMN/Cre ratio of 6299 (reference standard range: 7–124) were abnormally high. Urine samples were collected again at the 1-month postoperative check-up to measure urinary MN and NMN levels. The MN/Cre ratio was 24, and the NMN/Cre ratio was 266, which was lower than the preoperative values. Therefore, the resected lung tumor was diagnosed as catecholamine-producing neuroendocrine carcinoma. In this case, the lung tumor was diagnosed as a primary paraganglioma. The case is alive as of 580 days postoperatively, but at the owner's request, aggressive postoperative observation, including CT scans, has not been possible. The dog continues to show symptoms of Cushing's syndrome, but no respiratory symptoms. DiscussionIn this case, CT showed a left caudal lobar mass, enlarged middle tracheobronchial lymph nodes, and a right adrenal mass. The pulmonary mass was strongly enhanced in the venous phase and was washed out in the equilibrium phase. A previous study reporting the CT findings of paragangliomas in 10 dogs showed strong contrast enhancement in the arterial and venous phases (Gombert et al., 2022). The strong contrast-enhancing effect and subsequent washout of the left caudal lobar mass seen in this case are characteristic imaging findings that are suspicious for neuroendocrine tumors and are also seen in insulinomas and carcinoids, in addition to paragangliomas (Fukushima et al., 2016; Kutara et al., 2017). The resected lung mass was diagnosed as a neuroendocrine carcinoma by pathological examination, which was consistent with the imaging findings. In short, even in paragangliomas of the lung, this characteristic contrasting finding may help in decision-making. The enlarged right tracheobronchial lymph node showed the same contrast pattern as the mass and was suspected to be metastatic. Although pathological evaluation of the right adrenal mass was not performed in this case because it was not removed, it has been reported that adrenal masses can be diagnosed preoperatively using a triple-phase helical CT scan (Yoshida et al., 2016). In this case, the right adrenal mass was 10 mm in size with a smooth limbus and contrast enhancement at the limbus, peaking in the venous phase and diminishing in the equilibrium phase. Following previous reports, the right adrenal mass was considered an adrenal adenoma rather than a suspected pheochromocytoma. In this case, blood tests revealed elevated ALP levels, possibly due to the corticosteroids. Thus, based on these results, it was unlikely that the left lung mass was a metastatic lesion of the right adrenal mass and was considered a primary lung mass.
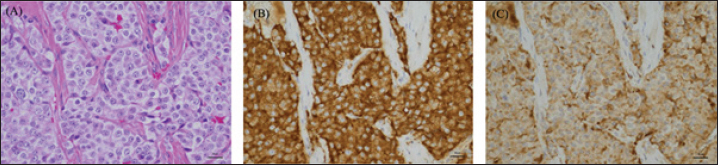
Fig. 4. High-power microscopic images of the pulmonary mass. (A) On H&E, the mass is composed of densely packed polyhedral cells compartmentalized by a fibrovascular stroma. The neoplastic cells have round nuclei and moderately abundant, eosinophilic granular cytoplasm. There is moderate anisokaryosis, and nuclear chromatin is coarsely granular with 1-2 small nucleoli. The neoplastic cells are diffusely positive for Synaptophysin (B) and Chromogranin A (C). Although the imaging findings in this case suggested a neuroendocrine tumor, a catecholamine-producing tumor was not suspected until a hypertensive crisis occurred during left pulmonary mass removal. Hypertensive crisis is a complication of pheochromocytoma and paraganglioma (Galac and Korpershoek, 2017; Ng et al., 2023). During mass removal, the dog suddenly developed severe hypertension due to catecholamine release. Nicardipine, a vasoselective calcium channel blocker, was adopted for antihypertensive rescue as an alternative to phenoxybenzamine, which was not available at that time. Nicardipine is commonly utilized for hypertensive urgency in the perioperative period as well as controlling hypertension induced by pheochromocytoma in human patients (Arai et al., 1986; Biassoni et al., 2020; Groeben et al., 2020). In dogs, there have been reports of the use of nicardipine for the antihypertensive treatment of experimentally induced hypertension, which has shown usefulness (Akashi et al., 2024). The present case indicated that nicardipine may successfully alleviate hypertension induced by catecholamine-producing neuroendocrine carcinoma also in dogs. The blood pressure stabilized after the removal was completed. The patient is still alive 580 days postoperatively. This is a survival time comparable to previous reports of paragangliomas and longer than that of pheochromocytoma of the lung. The urinary metanephrine (MN) and NMN levels measured in this case have been reported to help differentiate pheochromocytomas (Sasaki et al., 2021). The dog had a high MN/Cre ratio of 904 and an NMN/Cre ratio of 6,299, suggesting that this lung mass was a catecholamine-producing tumor. The dog also had a right adrenal mass, and the possibility of catecholamine release from the adrenal mass could not be ruled out. However, this was unlikely based on imaging findings. Therefore, the catecholamine level decreased when the patient was rechecked at the 1-month checkup after removing the lung mass. Thus, the catecholamine release associated with this intraoperative hypertensive crisis was thought to have occurred in the left lung mass. Based on these findings, the patient was diagnosed with primary paraganglioma of the lung. Postoperatively, the NMN/Cre ratio was mildly elevated to 266, which may reflect the metastatic findings of a pulmonary mass in the middle tracheobronchial lymph nodes. Regardless of their subtype, pulmonary tumors are often asymptomatic (Ogilvie et al., 1989). However, when neuroendocrine tumors are suspected on preoperative CT scans, urinary MN, and NMN levels can be measured preoperatively to evaluate catecholamine-producing potential. These preoperative examinations may predict the occurrence of hypertension due to intraoperative procedures, suggesting that they may contribute to safer perioperative management. Urinary MN and NMN levels should also be measured postoperatively to diagnose primary lung paragangliomas accurately. AcknowledgmentsPathological evaluation was performed at the Veterinary Pathology Diagnostic Center. We want to acknowledge Dr. Mika Tanabe (Diplomate, American College of Veterinary Pathologists) for the pathological diagnosis. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis research received no specific grant. Author’ contributionsConceptualization, Y. G., S. M., N. A., T. K., Y. O., and T. A.; methodology, S. M.; formal analysis, Y. G. and S. M.; investigation, S. M., N. A., T. K., K, K., and Y. O.; data curation, Y. G. and S. M.; writing—original draft preparation, Y. G.; writing—review and editing, Y. G., S. M., N. A., T. K., Y. O., and T. A.; supervision, S. M. and T. A. All authors have read and agreed to the published version of the manuscript. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAkashi, N., Murahata, Y., Tsuno, S., Kanazawa, A., Hikasa, Y. and Imagawa, T. 2024. Nicardipine constant rate infusion alleviates the cardiovascular effects of dexmedetomidine infusions without affecting the minimal alveolar concentration in sevoflurane-anesthetized dogs. Res. Vet. Sci. 172, 105254. Arai, T., Hatano, Y., Ishida, H. and Mori, K. 1986. Use of nicardipine in the anesthetic management of pheochromocytoma. Anesth. Analg. 65(6), 706–708. Barthez, P.Y., Marks, S.L., Woo, J., Feldman, E.C. and Matteucci, M. 1997. Pheochromocytoma in dogs: 61 cases (1984–1995). J. Vet. Intern. Med. 11, 272–278. Biassoni, R., Di Marco, E., Squillario, M., Barla, A., Piccolo, G., Ugolotti, E., Gatti, C., Minuto, N., Patti, G., Maghnie, M. and d'Annunzio, G. 2020. Gut microbiota in T1DM-onset pediatric patients: machine-learning algorithms to classify microorganisms as disease linked. J. Clin. Endocrinol. Metab. 105(9), 407. Brodey, R.S. and Craig, P.H. 1965. Primary pulmonary neoplasms in the dog: a review of 29 cases. J. Am. Vet. Med. Assoc. 147, 1628–1643. Choi, U.S., Alleman, A.R., Choi, J.H., Kim, H.W., Youn, H.J. and Lee, C.W. 2008. Cytologic and immunohistochemical characterization of a lung carcinoid in a dog. Vet. Clin. Pathol. 37, 249–252. Fukushima, K., Fujiwara, R., Yamamoto, K., Kanemoto, H., Ohno, K., Tsuboi, M., Uchida, K., Matsuki, N., Nishimura, R. and Tsujimoto, H. 2016. Characterization of triple-phase computed tomography in dogs with pancreatic insulinoma. J. Vet. Med. Sci. 77, 1549–1553. Galac, S. and Korpershoek, E. 2017. Pheochromocytomas and paragangliomas in humans and dogs. Vet. Comp. Oncol. 15, 1158–1170. Gombert, A., Diana, A., Hecht, S., Nicoli, S., Fracassi, F., Mortier, J., Reyes-Gomez, E. and Pey, P. 2022. Imaging features of retroperitoneal extra-adrenal paragangliomas in 10 dogs. Vet. Radiol. Ultrasound. 63, 393–402. Groeben, H., Walz, M.K., Nottebaum, B.J., Alesina, P.F., Greenwald, A., Schumann, R., Hollmann, M.W., Schwarte, L., Behrends, M., Rössel, T., Groeben, C., Schäfer, M., Lowery, A., Hirata, N., Yamakage, M., Miller, J.A., Cherry, T.J., Nelson, A., Solorzano, C.C., Gigliotti, B., Wang, T.S., Wietasch, J.K.G., Friederich, P., Sheppard, B., Graham, P.H., Weingarten, T.N. and Sprung, J. 2020. International multicentre review of perioperative management and outcome for catecholamine-producing tumours. Br. J. Surg. 107(2), e170–e178. Hu, S.P., Zhang, Z., Xiao, F., Huang, J.N., Jiang, Y., Mao, D.S., Wang, J.F. and He, X.J. 2022. Paraganglioma of the urinary bladder in a dog. J. Comp. Pathol. 195, 1–6. Ilha, M.R.S. and Styer, E.L. 2013. Extra-adrenal retroperitoneal paraganglioma in a dog. J. Vet. Diagn. Invest. 25, 803–806. Kutara, K., Konno, T., Kondo, H., Yamazoe, H. and Matsunaga, S. 2017. Triple-phase helical computed tomography of an arterio-hepatic venous shunt in a hepatic tumor in a dog. J. Vet. Med. Sci. 79, 1947–1951. Lunn, K.F. and Boston, S.E. 2020. Tumors of the endocrine system. In Withrow and MacEwen’s small animal clinical oncology, 6th ed. Eds., Vail, D.M., Thamm, D.H. and Liptak, J.M. St. Louis, MO: Elsevier, pp: 565–575. McPhetridge, J.B., Scharf, V.F., Regier, P.J., Toth, D., Lorange, M., Tremolada, G., Dornbusch, J.A., Selmic, L.E., Bae, S., Townsend, K.L., McAdoo, J.C., Thieman, K.M., Solari, F., Walton, R.A., Romeiser, J., Tuohy, J.L. and Oblak, M.L. 2021. Distribution of histopathologic types of primary pulmonary neoplasia in dogs and outcome of affected dogs: 340 cases (2010-2019). J. Am. Vet. Med. Assoc. 260, 234–243. Moulton, J.E., von Tscharner, C. and Schneider, R. 1981. Classification of lung carcinomas in the dog and cat. Vet. Pathol. 18, 513–528. Ng, D.Z., Than Yu, K.P. and Rajkanna, J. 2023. Acute pulmonary edema as a cardiovascular manifestation of pheochromocytoma. Cureus 15, e33675. Ogilvie, G.K., Haschek, W.M., Withrow, S.J., Richardson, R.C., Harvey, H.J., Henderson, R.A., Fowler, J.D., Norris, A.M., Tomlinson, J. and McCaw, D. 1989. Classification of primary lung tumors in dogs: 210 cases (1975-1985). J. Am. Vet. Med. Assoc. 195, 106–108. Park, Y.T. and Minamoto, T. 2021. Laparoscopic resection of retroperitoneal paraganglioma close to caudal vena cava in a dog. Vet. Med. Sci. 7, 2191–2197. Robat, C., Houseright, R., Murphey, J., Sample, S. and Pinkerton, M. 2016. Paraganglioma, pituitary adenoma, and osteosarcoma in a dog. Vet. Clin. Pathol. 45, 484–489. Rodrigues, F.R.N., da Silva Freire, J.M., Fidelis, L.A.P., Pereira, A.A.B.G., de Sousa, D.E.R., Wilson, T.M., Soto-Blanco, B. and de Castro, M.B. 2020. Paraganglioma of the tongue in a Chow Chow dog: a comparison with the human counterpart and literature review. Front. Vet. Sci. 7, 422. Saegusa, S., Yamamura, H., Morita, T. and Hasegawa, A. 1994. Pulmonary neuroendocrine carcinoma in a four-month-old dog. J. Comp. Pathol. 111, 439–443. Sasaki, N., Ikenaka, Y., Inoue, Y., Ichise, T., Nagata, N., Ishizuka, M., Nakayama, S.M., Nakamura, K. and Takiguchi, M. 2021. Urinary free metanephrines measurement in dogs with adrenal gland diseases using a new simple liquid chromatography tandem mass spectrometry method. J. Vet. Med. Sci. 83, 648–655. Tamura, J., Yoshida, S., Nagata, N., Shimbo, G. and Oyama, N. 2023. Successful treatment of acute respiratory failure following hypertensive crisis in a dog with presumed pheochromocytoma or paraganglioma. Open Vet. J. 13, 1465–1470. Wilson, T.M. and Soto-Blanco, B. 2020. Paraganglioma of the tongue in a Chow Chow dog: a comparison with the human counterpart and literature review. Front. Vet. Sci. 7, 422. Yanagawa, H., Hatai, H., Taoda, T., Boonsriroj, H., Kimitsuki, K., Park, C.-H. and Oyamada, T. 2014. A canine case of primary intra-right atrial paraganglioma. J. Vet. Med. Sci. 76, 1051–1053. Yoshida, O., Kutara, K., Seki, M., Ishigaki, K., Teshima, K., Ishikawa, C., Iida, G., Edamura, K., Kagawa, Y. and Asano, K. 2016. Preoperative differential diagnosis of canine adrenal tumors using triple-phase helical computed tomography. Vet. Surg. 45, 427–435. | ||
| How to Cite this Article |
| Pubmed Style Goda Y, Mizutani S, Akashi N, Kanda T, Kutara K, Okamura Y, Asanuma T. A case of primary pulmonary paraganglioma in a dog. Open Vet. J.. 2024; 14(10): 2714-2720. doi:10.5455/OVJ.2024.v14.i10.22 Web Style Goda Y, Mizutani S, Akashi N, Kanda T, Kutara K, Okamura Y, Asanuma T. A case of primary pulmonary paraganglioma in a dog. https://www.openveterinaryjournal.com/?mno=205268 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i10.22 AMA (American Medical Association) Style Goda Y, Mizutani S, Akashi N, Kanda T, Kutara K, Okamura Y, Asanuma T. A case of primary pulmonary paraganglioma in a dog. Open Vet. J.. 2024; 14(10): 2714-2720. doi:10.5455/OVJ.2024.v14.i10.22 Vancouver/ICMJE Style Goda Y, Mizutani S, Akashi N, Kanda T, Kutara K, Okamura Y, Asanuma T. A case of primary pulmonary paraganglioma in a dog. Open Vet. J.. (2024), [cited January 25, 2026]; 14(10): 2714-2720. doi:10.5455/OVJ.2024.v14.i10.22 Harvard Style Goda, Y., Mizutani, . S., Akashi, . N., Kanda, . T., Kutara, . K., Okamura, . Y. & Asanuma, . T. (2024) A case of primary pulmonary paraganglioma in a dog. Open Vet. J., 14 (10), 2714-2720. doi:10.5455/OVJ.2024.v14.i10.22 Turabian Style Goda, Yoshimichi, Shinya Mizutani, Natsuki Akashi, Teppei Kanda, Kenji Kutara, Yasuhiko Okamura, and Taketoshi Asanuma. 2024. A case of primary pulmonary paraganglioma in a dog. Open Veterinary Journal, 14 (10), 2714-2720. doi:10.5455/OVJ.2024.v14.i10.22 Chicago Style Goda, Yoshimichi, Shinya Mizutani, Natsuki Akashi, Teppei Kanda, Kenji Kutara, Yasuhiko Okamura, and Taketoshi Asanuma. "A case of primary pulmonary paraganglioma in a dog." Open Veterinary Journal 14 (2024), 2714-2720. doi:10.5455/OVJ.2024.v14.i10.22 MLA (The Modern Language Association) Style Goda, Yoshimichi, Shinya Mizutani, Natsuki Akashi, Teppei Kanda, Kenji Kutara, Yasuhiko Okamura, and Taketoshi Asanuma. "A case of primary pulmonary paraganglioma in a dog." Open Veterinary Journal 14.10 (2024), 2714-2720. Print. doi:10.5455/OVJ.2024.v14.i10.22 APA (American Psychological Association) Style Goda, Y., Mizutani, . S., Akashi, . N., Kanda, . T., Kutara, . K., Okamura, . Y. & Asanuma, . T. (2024) A case of primary pulmonary paraganglioma in a dog. Open Veterinary Journal, 14 (10), 2714-2720. doi:10.5455/OVJ.2024.v14.i10.22 |