| Research Article | ||
Open Vet. J.. 2024; 14(10): 2587-2598 Open Veterinary Journal, (2024), Vol. 14(10): 2587–2598 Research Article Prevalence of hemoparasitic infections and influencing factors among fighting bulls in Southern Thailand: A retrospective analysisWiruntita Bohman1, Nantaporn Chooruang2, Kitikarn Sakuna1, Wipaporn Jarujareet3, Kosit Areekit4 and Dhiravit Chantip3*1Department of Veterinary Clinical Sciences, Faculty of Veterinary Sciences, Rajamangala University of Technology Srivijaya, Nakhon Si Thammmarat, Thailand 2Laboratory and Diagnostic Centre of the Teaching Animal Hospital and Community Services, Faculty of Veterinary Sciences, Rajamangala University of Technology Srivijaya, Nakhon Si Thammmarat, Thailand 3Department of Veterinary Bio-Medical Sciences, Faculty of Veterinary Sciences, Rajamangala University of Technology Srivijaya, Nakhon Si Thammmarat, Thailand 4Livestock Animal Hospital, Faculty of Veterinary Sciences, Rajamangala University of Technology Srivijaya, Nakhon Si Thammmarat, Thailand *Corresponding Author: Dhiravit Chantip. Department of Veterinary Bio-Medical Sciences, Faculty of Veterinary Sciences, Rajamangala University of Technology Srivijaya, Nakhon Si Thammmarat, Thailand. Email: dhiravit.c [at] rmutsv.ac.th Submitted: 17/06/2024 Accepted: 22/09/2024 Published: 31/10/2024 © 2024 Open Veterinary Journal
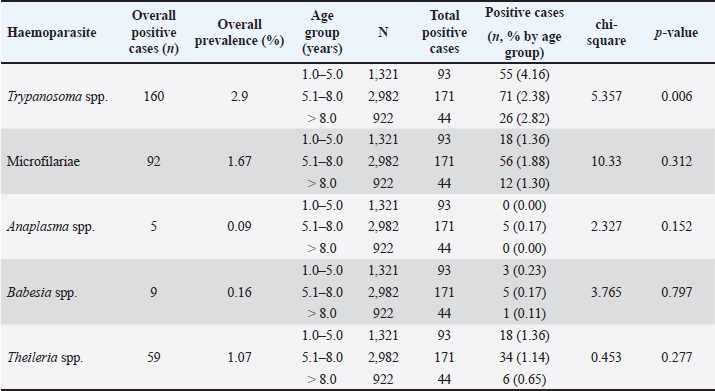
AbstractBackground: Hemoparasitic infections significantly threaten cattle health globally, leading to economic losses and welfare issues for farmers. Aim: This retrospective study aimed to investigate the prevalence and associated factors of hemoparasitic infections in fighting bulls in southern Thailand over an 8-year period. Methods: Laboratory records of 5,518 bulls from the Livestock Animal Hospital, Faculty of Veterinary Science, Rajamangala University of Technology Srivijaya, were reviewed for the period January 2016 to December 2023. Blood samples were analyzed using thin blood smear methods to identify hemoparasites, and packed cell volume was measured using the microhematocrit method. Detailed profiles and demographic data for each animal were recorded. Results: Among the fighting bulls, 323 (5.85%) tested positive for hemoparasitic infections, with five hemoparasites identified: Trypanosoma spp. (TP), Microfilariae, Anaplasma spp. (AP), Babesia spp. (BB), and Theileria spp. (TR). TP was the most prevalent at 2.90%. Bulls aged 1–5 years showed the highest infection rates at 7.04%, with a decline in infections as age increased. Annual trends peaked in 2018 at 8.80%, with significant yearly fluctuations for most parasites (p < 0.05) except AP and BB. Monthly analyses revealed the highest infection rates in March, particularly for TP, with significant monthly variations for TR (p < 0.05). Geographical differences in infection rates between the East and West coasts were minor and not statistically significant. However, seasonal variations were significant for BB and TR on the east coast during summer (p < 0.05). Bulls with PCV below 30% had a higher prevalence of hemoparasitic infections (8.06%) compared to those with PCV above 30% (5.87%), with significant differences in the prevalence of AP and TR infections (p < 0.05). Conclusion: This study adds to the understanding of hemoparasite infections in bulls, informing the development of educational materials for farmers and ultimately empowering them to make better herd health decisions. Keywords: Blood parasite, Prevalence, Indigenous cattle, Bull, Epidemiological factors. IntroductionHemoparasitic infections in cattle are critically important due to their significant impact on livestock health, productivity, and the broader agricultural economy (Heylen et al., 2023). Infections caused by protozoan and rickettsial parasites such as Babesia (BB), Theileria (TR), Anaplasma (AP), and Trypanosoma (TP) species can lead to diseases with varying severity, manifesting as anemia, fever, weight loss, and sometimes death (Tefi et al., 2015; Julie et al., 2021). Morbidity rates for hemoparasitic infections in cattle can be as high as 90%, with mortality rates ranging from 10% to over 50%, influenced by the parasite species, cattle breed, and local conditions (Swai et al., 2009; Khan et al., 2021). These parasites are predominantly transmitted by ticks, with various tick species acting as vectors for different hemoparasites (Yitayew and Samuel, 2015). The economic impact is considerable, encompassing reduced meat and milk production, increased veterinary expenses, and potential trade restrictions (Strydom et al., 2023). The prevalence of hemoparasitic infections in cattle varies widely across regions, especially in tropical areas (Ferraz da Costa et al., 2014; Jirapattharasate et al., 2017; Dharanesha et al., 2017). This variability is influenced by factors such as climate, vector presence, management practices, and host immunity (Maharana et al., 2016; Weny et al., 2017). Fighting bulls, indigenous cattle in southern Thailand, are selected for bullfighting due to their superior physical attributes, including body conformation, skin pigmentation, horn structure, aggressive behavior, and proficient fighting skills. This culturally significant sport has grown in popularity, attracting tourists and boosting local economies (Mhonghong et al., 2019; Chodchaey et al., 2022). Bulls chosen for competitions typically undergo rigorous training and conditioning from the age of 4–5 years, involving daily walks of 5–10 km and monthly sparring sessions to achieve peak physical fitness (Mhonghong et al., 2019; Chodchaey et al., 2022). Given their high value, effective health monitoring and management are crucial for ensuring the robustness and enhancing the fighting capabilities of these bulls. Hemoparasitic infections in beef and dairy cattle in Thailand have been extensively investigated, revealing the presence of various hemoparasites, including BB, TR, and AP, in the central, western, and northern regions of the country. High incidences of these infections are reported in areas with dense cattle populations (Kaewthamasorn and Wongsamee, 2006; Jirapattharasate et al., 2017; Srionrod et al., 2022; Koonyosying et al., 2022). A previous study reported a 42.50% prevalence of hemoparasitic infections in fighting bulls (Keawchana et al., 2021). However, limited knowledge exists on hemoparasitic infections in fighting bulls, particularly regarding their prevalence, temporal patterns, and potential risk factors. This study aimed to address this gap by investigating the prevalence and temporal dynamics of these infections in fighting bulls from southern Thailand over an 8-year period. Additionally, it sought to identify and analyze factors associated with these infections within this unique population, with the aim of improving individual and herd health management practices. Materials and MethodsStudy areaBlood samples were collected from fighting bulls at the Large Animal Hospital (or relevant department) within the Faculty of Veterinary Science, Rajamangala University of Technology Srivijaya (RUTS), Nakhon Si Thammarat campus, located within the latitudinal range of 5°–12° North and longitudes 98°–103° East. These bulls originated from various regions across southern Thailand. The region experiences a tropical monsoon climate with a longer rainy season on the west coast (April-November) compared to the east coast (May-December). Annual rainfall is higher on the west coast (3,000 mm) compared to the east coast (2,000 mm). Temperatures range from 23°C to 33°C throughout the year with slightly higher humidity on the east coast (75%–90%) compared to the west coast (70%–85%). The geographical distribution of the participating fighting bulls across southern Thailand is presented in Figure 1. Data extractionConcerning the data extraction, blood samples from all cases of fighting bulls brought to the Livestock Animal Hospital at the Faculty of Veterinary Science, RUTS, Nakhon Si Thammarat campus, Thailand, for individual health examinations, with or without clinical signs related to hemoparasitic infections between January 2016 and December 2023, were reviewed and carefully analyzed. The demographic data collected from the database, including the age and other demographic information of the bulls, were systematically recorded for all study subjects. Sample collection and analysisBlood samples collected from the jugular veins of 5,518 fighting bulls were prepared as thin blood smears and stained with a 10% Giemsa solution for 30 minutes. The stained slides were then examined for hemoparasites under a light microscope at 100 × oil immersion by an experienced laboratory technician at the Laboratory and Diagnostic Center of the Teaching Animal Hospital and Community Services, Faculty of Veterinary Science, RUTS. Additionally, packed cell volume (PCV) was measured using the microhematocrit centrifuge method, and the PCV ranges were categorized into two groups: below 30% and above 30%. Statistical analysisData collected over the course of the study period were subjected to analysis using IBM® SPSS Statistics version 20 software. The nonparametric chi-square test was employed to evaluate the associations between various variables, including age, duration of the study, geographic and provincial locations of farming, seasonal variations and PCV ranges, and the incidence of blood parasite infections, with a confidence level of 95%. Ethical approvalThis retrospective study received approval from the Institutional Animal Care and Use Committee of the University of Technology Srivijaya, Thailand (Approval No. IAC 05-09-2024). ResultsThe comprehensive results of the 8-year (2016-2023) monitoring of hemoparasitic infections among fighting bulls in southern Thailand are presented in Table 1. The screening identified the presence of TP, Microfilariae (MC), TR, BB, and AP. The overall prevalence of these hemoparasites was 5.85%. TP was the most frequently detected parasite, identified in 160 cases, corresponding to a prevalence rate of 2.90%. This was followed by MC, found in 92 bulls (1.67%), and TR, identified in 59 animals (1.07%). BB and AP were the least prevalent, detected in 9 (0.16%) and 5 (0.09%) cases, respectively.
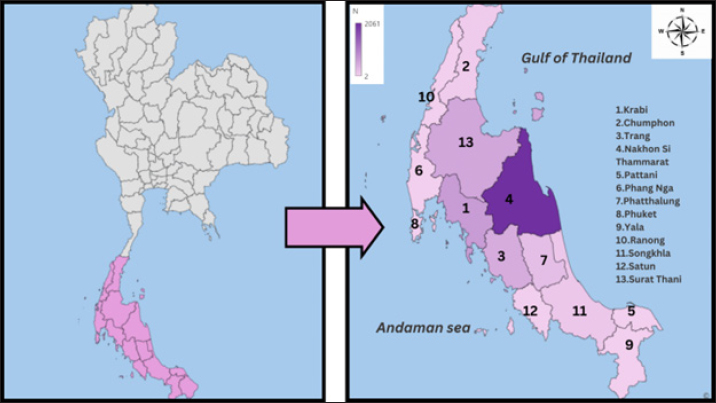
Fig. 1. Geographical origin of fighting bulls in this study. Table 1. Prevalence of hemoparasites in fighting bulls by age group and overall (n=5,518).
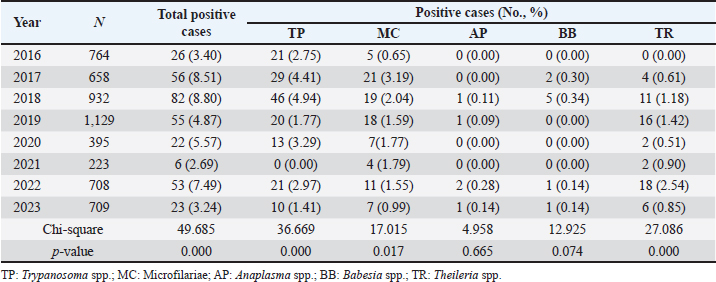
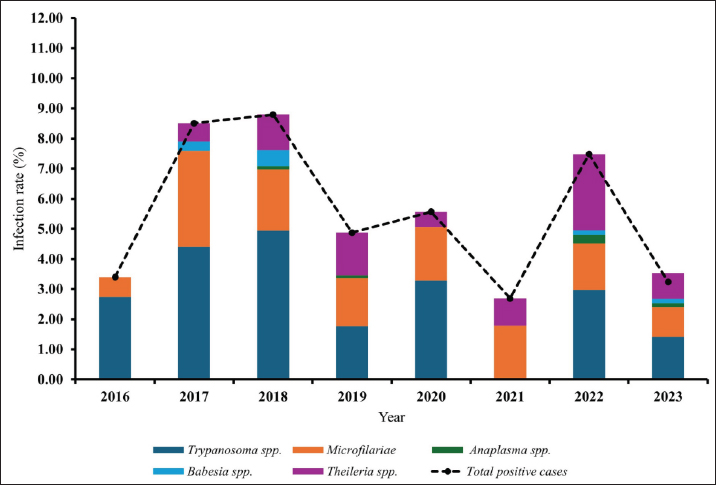
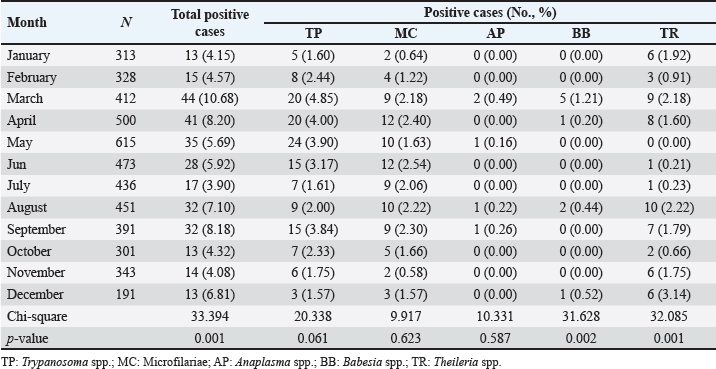
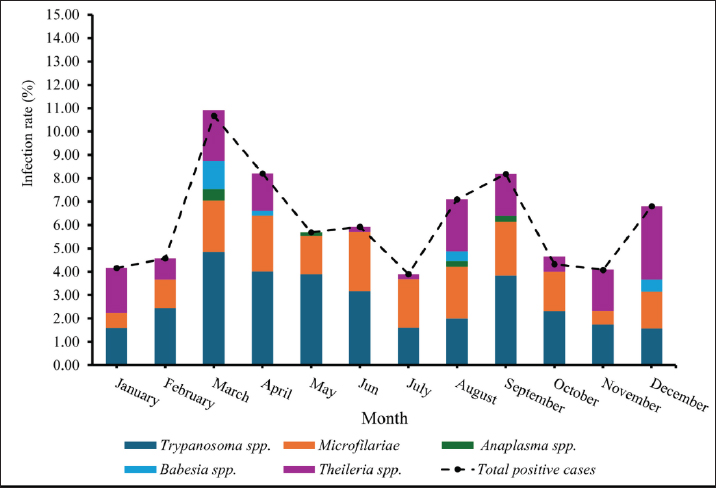
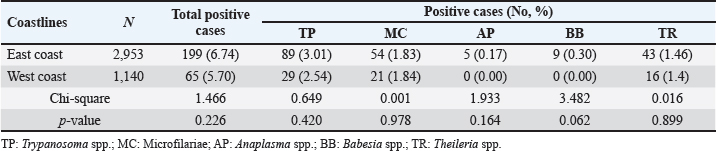
Age-wised hemoparasitic infectionsThe association between the age group and the prevalence of hemoparasitic infections was notable (p=0.069). The highest prevalence of hemoparasitic infections was observed in the 1.0–5.0 years age group (7.04%) (Table 1). TP was most prevalent in this youngest age group, with 55 cases, representing an infection rate of 4.16%. The prevalence decreased with age, reaching its lowest in the oldest age group (>8.0 years) at 2.82%. The variation in prevalence among age groups was statistically significant (p < 0.05). MC was also more prevalent in the youngest group, with 18 cases (1.36%), and its prevalence decreased with age, showing 1.30% in the oldest group. However, the differences among age groups for MC were not statistically significant. AP and BB were less frequently detected, with the highest prevalence observed in the middle age group (5.1–8.0 years) at 1.88% for AP and 0.17% for BB, showing no significant variation across age groups. TR exhibited a slightly higher prevalence in the youngest age group at 1.36%, and the lowest in the oldest group at 0.65%. The variations in TR prevalence across age groups were not statistically significant. Year-wised hemoparasitic infectionsSignificant variations in the prevalence rates were observed over the years for TP, MC, and TR (p < 0.05), while AP and BB did not exhibit significant fluctuations (Table 2). The overall trend indicated an initial increase in infection rate, peaking in 2018, followed by a decline, and then a modest resurgence in 2022 (Fig. 2). The highest prevalence was observed in 2018 (8.80%), whereas the lowest prevalence occurred in 2021 (2.69%). Specifically, the prevalence of TP was highest in 2018 with 46 positive cases (4.94%), and lowest in 2021 with no reported cases. MC infections showed a relatively consistent presence across the years, peaking in 2017 with 21 positive samples (3.19%). The prevalence of TR varied, reaching a high of 18 positive cases (2.54%) in 2022. Month-wised hemoparasitic infectionsOverall, hemoparasitic infections exhibited the highest prevalence rate in March, with 44 positive cases (10.68%), and demonstrated significant monthly variations (p < 0.05), as shown in Table 3 and Figure 3. TP had fluctuating prevalence rates throughout the year, peaking in March with 20 cases (4.85%), although the statistical analysis indicated that these monthly variations tend to be significant (p=0.06). MC showed higher occurrences in June and August, with 12 cases each (2.54% and 2.22%, respectively), also indicating no significant monthly variation. AP presented a lower prevalence overall, with the highest number of cases in March and August (2.18% and 2.22%, respectively), but these fluctuations were not statistically significant. BB was detected the least frequently, with a few cases scattered across the months, and the chi-square test revealed significant variations, mainly due to the very low number of cases (p < 0.05). TR exhibited a distinct peak in December, with 6 cases (3.14%), and the monthly variations in prevalence were significant (p < 0.05). Hemoparasitic infections in various coastal regionsThe prevalence of hemoparasitic infections among fighting bulls in various coastal regions is presented in Table 4. TP exhibited a slightly higher prevalence on the East Coast, with 89 positive cases (3.01%), compared to the West Coast, which recorded 29 cases (2.54%). However, the difference in prevalence between the two coastlines was not statistically significant. MC showed similar prevalence rates on both coastlines, with 1.83% on the east coast and 1.84% on the west coast, indicating no significant difference. AP was notably rare on the West Coast, with no cases reported, while a small number of cases (5 cases, 0.17%) were observed on the East Coast, yet statistical analysis revealed no significant variation. BB was detected only on the east coast, with 9 cases (0.30%), and none on the west coast, showing a marginally non-significant difference. TR had a prevalence of 1.46% on the East Coast and 1.4% on the West Coast, with no significant difference between the locations. Table 2. Prevalence of hemoparasitic infections in fighting bulls in southern Thailand from 2016 to 2023, shown as positive cases and percentages.
Fig. 2. The infection rate of hemoparasitic infections in fighting bulls from 2016 to 2023. Table 3. Monthly prevalence of hemoparasitic infections in fighting bulls, reported as positive cases and percentages.
Fig. 3. Monthly distribution of hemoparasitic infections in fighting bulls. Table 4. The prevalence of hemoparasitic infections on different coastlines in southern Thailand, reported as positive numbers and percentages.
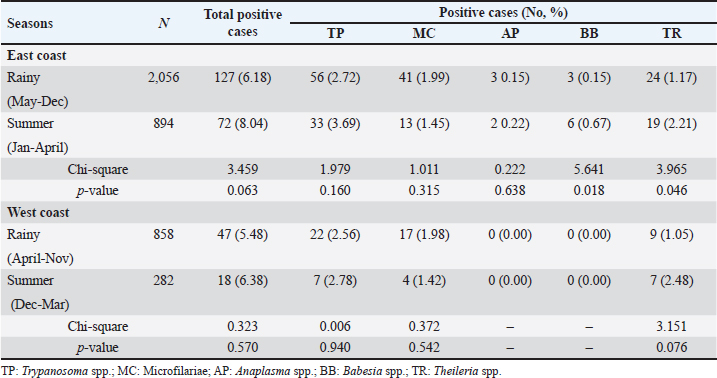
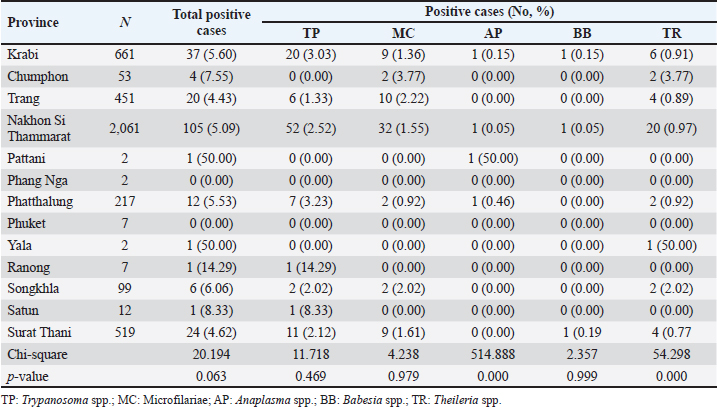
Season-wised hemoparasitic infectionsOur results showed significant variations in the prevalence of BB and TR during different seasons on the East Coast (p < 0.05). BB and TR showed higher infection rates during the summer (0.67% and 2.21%) than in the rainy season (0.15% and 1.17%). On the West Coast, there were no significant seasonal differences in the prevalence of the tested parasites, except for TR, which approached significance with a higher infection rate during the rainy season than in the summer (Table 5). Hemoparasitic infections observed in distinct provincesThe results revealed significant geographical variations in infection rates (Table 6). TP had the highest prevalence in Krabi (3.0%) and the highest number of cases in Nakhon Si Thammarat (2.5%). MC infections were most common in Krabi (1.4%) and Nakhon Si Thammarat (1.6%), with other provinces reporting lower rates. AP was rarely detected, with minimal cases primarily in Nakhon Si Thammarat. BB was found exclusively in Nakhon Si Thammarat and Krabi. TR had sporadic occurrences, mainly in Krabi and Phatthalung (0.9%). Statistical analysis discovered significant variations in the prevalence of TR and BB, but not for MC, AP, and TR in substantial regional differences. Table 5. Seasonal prevalence of hemoparasitic infections on both coastlines (East and West) in southern Thailand, shown as positive cases and percentages.
Table 6. The prevalence of hemoparasitic infections in the provinces of southern Thailand, showing the number of positive cases and their corresponding percentages.
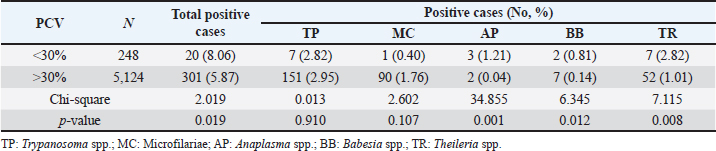
Table 7. The prevalence of hemoparasitic infections in fighting bulls, reported as positive cases and percentages, categorized by pack cell volume ranges.
PCV-wised hemoparasitic infectionsThe findings on the prevalence of hemoparasitic infections in fighting bulls, categorized by PCV ranges are represented in Table 7. The results indicated a higher prevalence of hemoparasitic infections in bulls with PCV below 30% (8.06%) compared to those with PCV over 30% (5.87%). Notably, the prevalence of AP, BB, and TR infections showed significant differences between the two PCV groups (p < 0.05). DiscussionComprehensive monitoring of hemoparasitic infections among fighting bulls in southern Thailand from 2016 to 2023 revealed an overall prevalence of 5.85%, with TP being the most frequently detected parasite. This contrasts with previous studies, such as Keawchana et al. (2021), which reported a much higher prevalence of 42.50% and identified TR as the most common parasite in bulls showing clinical signs of hemoparasitic infections. Another study by Koonyosying et al. (2022) highlighted regional differences, showing TR orientalis and AP marginale as the most prevalent in central and northern Thailand, with prevalence rates of 36.50% and 34.15%, respectively. Our findings of a lower infection rate may be attributed to fighting bulls having a higher tolerance to infection compared to other cattle breeds. Additionally, fighting bulls mostly receive intensive individual healthcare, unlike beef cattle raised on farms. Discrepancies in findings could also be due to differences in environmental conditions, cattle management practices, and diagnostic methods. These results emphasize the need for ongoing surveillance and region-specific interventions, underscoring the importance of tailored strategies to effectively manage hemoparasitic infections in cattle across Thailand. The age-related analysis of hemoparasitic infections in fighting bulls reveals critical trends for targeted control strategies. The highest prevalence was observed in the youngest age group (1.0–5.0 years), particularly for TP, likely due to the development of protective immunity in endemic areas, where young animals show asymptomatic infection, while older animals have a higher probability of being positive with symptoms. However, this data was not analyzed. Prevalence decreased with age, reaching its lowest in bulls over 8.0 years, suggesting acquired immunity. MC was more common in younger bulls, while AP and BB were more prevalent in middle-aged bulls, indicating the influence of environmental exposure and management practices. TR had a slightly higher prevalence in the youngest group without significant age variation. These findings underscore the need for age-specific management strategies, including enhanced preventive measures for younger bulls and consistent monitoring for older ones. Supporting studies indicate that cattle over 1-year-old are more susceptible to A. marginale in Côte d’Ivoire and Morocco, with higher rates in 1–3-year-olds and those over 3 years (Yéo et al., 2020). In Algeria, younger cattle (6 months to 1 year) showed higher infection rates for theileriosis (Ayadi et al., 2016). Hemoparasitic infections were more common in Holstein Friesian and Jersey crossbreeds than in indigenous breeds, with higher frequency in crossbred animals aged 2–7 years and in indigenous animals under 2 years old (Velusamy et al., 2014). The highest prevalence among male cattle was observed in those less than a year old, while the highest prevalence of hemoprotozoans in female cattle was in those of third parties (Subapriya et al., 2021). The observed age-specific patterns underscore the importance of tailored infection management strategies for young fighting bulls. These strategies should include routine prophylactic treatments, vaccinations (Bastos et al., 2020), and nutritional interventions to bolster their immune systems. Despite their potential for future competitive value, the health of young fighting bulls is frequently neglected. By implementing the findings of this study, targeted health management strategies can be developed to significantly improve the overall well-being of these animals and lay the foundation for long-term health and performance.Our study revealed that hemoparasitic infections in fighting bulls in southern Thailand vary seasonally and regionally. TP was more prevalent on the east coast, while MC had similar rates on both coasts. AP was rare on the West Coast, and BB was exclusive to the East Coast. Seasonally, BB and TR had higher rates on the East Coast during the summer, whereas TR had higher rates on the West Coast during the rainy season. These findings underscore the significant impact of geographical and seasonal factors on infection rates. Environmental conditions, such as increased vegetation and water availability during the rainy season, particularly with high levels of rainfall on the West Coast, likely support the populations of Ixodid ticks belonging to the genera Hyalomma and Rhipicephalus. These conditions facilitate higher transmission rates of theileriosis (Ali et al., 2013; Ayadi et al., 2016; Sarasombath et al., 2019). Similarly, the hot and humid summer conditions on the east coast may create an ideal environment for tabanid flies, which have recently been reported as vectors transmitting both BB and TR species in this region of southern Thailand (Sontigun et al., 2022). This environmental factor could potentially lead to higher infection rates. Moreover, the present study revealed significant yearly variations in the prevalence rates of TP, MC, and TR, while AP and BB did not show significant fluctuations. Monthly analysis highlighted notable seasonal variations, with overall hemoparasitic infections peaking in March. Specifically, TP and AP increased in March, MC peaked in June and August, BB appeared sporadically, and TR peaked in December. These variations are possibly due to changes in temperature, rainfall, and relative humidity during seasonal transitions, which create favorable conditions for the increased activity and population of hemoparasite vectors. These findings highlight the significant influence of climatic factors on the distribution and dynamics of hemoparasitic infections among fighting bulls. Previous research has shown fluctuations in the prevalence of vectors such as Rhipicephalus microplus, Haematobia irritans, and Dermatobia hominis, with the highest tick density in midwinter and peaks in fly and larvae populations correlating with rainfall in Brazil (Ferraz da Costa et al., 2014). Similarly, studies in Iraq and India have found variable prevalence rates of AP, BB, and TR influenced by temperature and rainfall (Abdullah et al., 2019; Jayalakshmi et al., 2019). In Karnataka, India, peaks in hemoparasitic infections in August and May were linked to specific climatic conditions such as the monsoon season (Dharanesha et al., 2017). These results underscore the necessity for region- and season-specific control strategies for hemoparasitic infections, particularly vector control, in fighting bulls. This approach should consider the influence of rainfall, temperature, and relative humidity on hemoparasite occurrences (Dharanesha et al., 2017; Abdullah et al., 2019; Hosen et al., 2020; Subapriya et al., 2021; Kumari et al., 2022; Shit et al., 2023). In Thailand and Southeast Asia, the widespread application of synthetic acaricides and insecticides (Artchayasawat et al., 2020; Tan et al., 2021; Lorn et al., 2022) has been a key component of vector control efforts to mitigate hemoparasite infections in cattle. However, the emergence of vector resistance has necessitated a paradigm shift in control strategies. A comprehensive training program for bull farmers, coupled with the exploration of organic alternatives and anti-tick vaccines (Jittapalapong et al., 2004), presents a promising avenue for the sustainable management of vector populations among fighting bulls in Southern Thailand. Geographical variations in hemoparasitic infections were observed among provinces in southern Thailand. TP exhibited the highest prevalence in Krabi, with the most cases reported in Nakhon Si Thammarat, while MC was commonly found in both provinces. AP was rare and primarily detected in Nakhon Si Thammarat, whereas BB was exclusive to Nakhon Si Thammarat and Krabi. TR infections occurred sporadically, mainly in Krabi and Phatthalung, with significant regional differences noted. These provinces have a high density of fighting bull populations and numerous training rings and bullfighting arenas, suggesting that bullfighting activities may contribute to the spread of infections. Previous studies have similarly indicated high rates of TR infection in Nakhon Si Thammarat and Songkhla (Keawchana et al., 2021). Therefore, the implementation of robust insect control programs and sanitation measures around training rings and bullfighting arenas to reduce vector populations should be considered. Hemoparasite infections caused by AP, BB, and TR resulted in the destruction of erythrocytes within the liver and spleen, leading to a significant reduction in red blood cell (RBC) indices such as RBC count, hemoglobin, and PCV (Saleh, 2009; Hamid et al., 2014; Pandey et al., 2017). In this study, a strong correlation was observed between PCV levels and the prevalence of these infections in fighting bulls. The correlation between PCV values and infection prevalence in this study aligns with previous findings (Hamid et al., 2014; Paul et al., 2016; Weerasooriya et al., 2016; Rakwong et al., 2022; Shit et al., 2023). Specifically, PCV levels below 30% were associated with a more pronounced negative impact from these hemoparasites, suggesting the potential utility of PCV as a prognostic marker for susceptibility to and severity of these infections. LimitationsWhile the study provides valuable insights into hemoparasitic infections among fighting bulls in southern Thailand, several limitations must be acknowledged. The exclusive focus on the southern region may limit generalizability, and sampling bias is possible as samples were collected from specific veterinary facilities. Microscopy-based diagnostics may lack the sensitivity of molecular techniques, potentially leading to underreporting. The study did not extensively explore coinfections or control for variations in management practices. Addressing these limitations in future research could enhance understanding and improve control and prevention strategies for hemoparasitic infections. ConclusionThis 8-year study of hemoparasitic infections in fighting bulls in southern Thailand provides valuable insights into their prevalence, distribution, and risk factors. The overall infection rate was 5.85%, with TP being the most prevalent parasite. Younger bulls, particularly those aged 1–5 years, exhibited higher infection rates. Annual and monthly trends, as well as geographical and seasonal variations, were observed, suggesting a complex interplay of factors influencing parasite transmission. Bulls with a PCV below 30% were at significantly higher risk of infections and experienced more severe health issues, emphasizing the potential value of PCV as a predictive marker. Targeted interventions are crucial for effective management of hemoparasitic infections in this region. Regular screening programs for young bulls and animals with low PCV, coupled with targeted vector control measures and the development of vaccines against TP and other prevalent hemoparasites, offer promising avenues for mitigating the impact of these infections on bull health and productivity. AcknowledgmentsThe authors would like to express their gratitude to the Director of the Teaching Animal Hospital and Community Services, and the Dean of the Faculty of Veterinary Science at RUTS for providing permission and supplying the necessary resources and data needed for the research project. Conflicts of interestIt is declared by the authors that no conflicts of interest exist. FundingThis research received no specific grant. Authors’ contributionsWB: Designed the study, conducted data analysis and interpretation, and drafted the manuscript., NC: Recorded data, performed hemoparasite detection and identification., KS: Designed the study and performed data analysis., WJ: Collected and assembled data., KA: Collected samples and recorded data., DC: Developed the research concept and revised the manuscript. All authors have read, reviewed, and approved the final manuscript. Data availabilityAll data are provided in the manuscript. ReferencesAbdullah, D.A., Ali, M.S., Omer, S.G., Ola-Fadunsin, S.D., Ali, F.F. and Gimba, F.I. 2019. Prevalence and climatic influence on hemoparasites of cattle and sheep in Mosul, Iraq. J. Adv. Vet. Anim. Res. 6(4), 492. Ali, Z., Maqbool, A., Muhammad, K., Khan, M.S. and Younis, M. 2013. Prevalence of theileria annulata infected hard ticks of cattle and buffalo in Punjab, Pakistan. J. Anim. Plant Sci. 23(1), 20–26. Artchayasawat, A., Boueroy, P., Boonmars, T., Pumhirunroj, B., Sriraj, P., Aukkanimart, R., Boonjaraspinyo, S., Pitaksakulrat, O., Ratanasuwan, P., Suwannatrai, A. and Eamudomkarn, C. 2020. The effects of water submersion on cattle ticks. Thai. J. Vet. Med. 50(3), 371–379. Ayadi, O., Rjeibi, M.R., Elfegoun, M.C.B. and Gharbi, M. 2016. Prevalence and risk factors of tropical theileriosis, and sequencing of Theileria annulata, the causative pathogen, in Setif region (Algeria) before and after tick season. Rev. Elev. Med. Vet. Pays. Trop. 69, 161–166. Bastos, T.S.A., Faria, A.M., de Assis Cavalcante, A.S., de Carvalho Madrid, D.M., Zapa, D.M.B., Nicaretta, J.E., Cruvinel, L.B., Heller, L.M., Couto, L.F.M. and Soares, V.E. 2020. Comparison of therapeutic efficacy of different drugs against Trypanosoma vivax on experimentally infected cattle. Prev. Vet. Med. 181, 105040. Chodchaey, T., Damrongwattana, J., Prathum, B., Onchun, P. and Suriya, S.P. 2022. Knowledge of bull fighting raising: a case study Ban Naimong community, Prom Lok sub-district, Promkiri district, Nakhon si Thammarat province. JMCU Phetchaburi Rev. 5, 50–69. Dharanesha, N.K., Giridhar, P., Byregowda, S.M., Venkatesh, M.D. and Ananda, K.J. 2017. Seasonal prevalence of blood parasitic diseases in crossbred cattle of Mysore and its surrounding districts of Karnataka. J. Parasit. Dis. 41, 773–777. Ferraz da Costa, M. do S., Guimarães, M.P., Lima, W. dos S., Ferraz da Costa, A.J., Facury Filho, E.J. and Araujo, R.N. 2014. Seasonal variation and frequency distribution of ectoparasites in crossbreed cattle in Southeastern Brazil. J. Vet. Med. 2014, 759854. Hamid, O.M.A., Radwan, M.E.I. and Ali, A.F. 2014. Biochemical changes associated with anaplasma infection in cattle. Glob. J. Biotechnol. Biochem. 9, 19–23. Heylen, D.J.A., Kumsa, B., Kimbita, E., Frank, M.N., Muhanguzi, D., Jongejan, F., Adehan, S.B., Toure, A., Aboagye-Antwi, F., Ogo, N.I., Juleff, N., Crafford, D., Fourie, J., Labuchange, M. and Madder, M. 2023. Tick-borne pathogens and body condition of cattle in smallholder rural livestock production systems in East and West Africa. Parasit. Vectors. 16(1), 117. Hosen, M., Jewel, A.B., Chowdhury, S.R., Uddin, B., Hossain, M., Rahman, M. and Rahman, M. 2020. Prevalence of haemoprotozoan diseases in cattle population in sylhet district of Bangladesh. Adv. Anim. Vet. Sci. 8, 17582. Jayalakshmi, K., Sasikala, M., Veeraselvam, M., Venkatesan, M., Yogeshpriya, S., Ramkumar, P.K., Selvaraj, P. and Vijayasarathi, M.K. 2019. Prevalence of haemoprotozoan diseases in cattle of Cauvery delta region of Tamil Nadu. J. Parasit. Dis. 43(2), 308–312. Jittapalapong, S., Jansawan, W., Barriga, O.O. and Stich, R.W. 2004. Reduced incidence of Babesia bigemina infection in cattle immunized against the cattle tick, Boophilus microplus. Ann. N. Y. Acad. Sci. 1026(1), 312–318. Jirapattharasate, C., Adjou Moumouni, P.F., Cao, S., Iguchi, A., Liu, M., Wang, G., Zhou, M., Vudriko, P., Efstratiou, A., Changbunjong, T., Sungpradit, S., Ratanakorn, P., Moonarmart, W., Sedwisai, P., Weluwanarak, T., Wongsawang, W., Suzuki, H. and Xuan, X. 2017. Molecular detection and genetic diversity of bovine Babesia spp., Theileria orientalis, and Anaplasma marginale in beef cattle in Thailand. Parasitol. Res. 116(2), 751–762. Julie, B., Subapriya, S. and Vairamuthu, S. 2021. Haematobiochemical changes in subclinical hemoprotozoan infections in bovine. J. Entomol. Zool. Stud. 9(1), 1764–1766. Kaewthamasorn, M. and Wongsamee, S. 2006. A preliminary survey of gastrointestinal and haemoparasites of beef cattle in the tropical livestock farming system in Nan Province, northern Thailand. Parasitol. Res. 99(3), 306–308. Keawchana, N., Rakwong, P. and Ngasaman, R. 2021. Haemoparasites infection in bullfighting cattle in southern of thailand. Vet. Integr. Sci. 9(2), 133–140. Khan, A., Ali, A., Jamil, M., Zeb, S., Arshad, S., Noman, M., Khan, I., Safiullah Ullah, A., Zeeshan, M., Ashraf, U. and Tariq, A. 2021. A review of theileria incidence in cattle population, its impact on hematology of the infected animals and therapeutic approach towards the infection. J. Crit. Rev. 8(3), 53–66. Koonyosying, P., Rittipornlertrak, A., Chomjit, P., Sangkakam, K., Muenthaisong, A., Nambooppha, B., Srisawat, W., Apinda, N., Singhla, T. and Sthitmatee, N. 2022. Incidence of hemoparasitic infections in cattle from central and northern Thailand. PeerJ. 10, e13835. Kumari, P., Minz, S.P., Sahay, S. and Das, S.S. 2022. Influence of weather variables on cattle diseases in Ranchi, Jharkhand. J. Agrometeorol. 24, 417–419. Lorn, S., Klakankhai, W., Nusen, P., Sumarnrote, A. and Tainchum, K. 2022. Pyrethroid susceptibility in Stomoxys calcitrans and Stomoxys indicus (Diptera: Muscidae) collected from cattle farms in southern Thailand. Insects 13(8), 711. Maharana, B.R., Kumar, B., Prasad, A., Patbandha, T.K., Sudhakar, N.R., Joseph, J.P. and Patel, B.R. 2016. Prevalence and assessment of risk factors for haemoprotozoan infections in cattle and buffaloes of South-West Fujarat, India. Indian J. Anim. Res. 50(5), 10268. Mhonghong, T., Dumrongwattana, J., Dechochai, U. and Kaenhamkaew, D. 2019. Fighting-bull school and folk wisdom of management the fighting-bull sport camp for commercial competition, a case study: fighting-bull stadium in Pagpol village, Napakhoe, Bangaew, Patthalung. JSC 3, 54–67. Pandey, V., Nigam, R., Bachan, R., Sudan, V., Jaiswal, A.K., Shankar, D., Kumar, R., Mandil, R. and Yadav, B. 2017. Oxidative and haemato-biochemical alterations in theileriosis affected cattle from semi-arid endemic areas of India. Indian J. Anim. Sci. 87(7), 846–850. Paul, B.T., Bello, A.M., Ngari, O., Mana, H.P., Gadzama, M.A., Abba, A., Malgwi, K.D., Balami, S.Y., Dauda, J. and Abdullahi, A.M. 2016. Risk factors of haemoparasites and some haematological parameters of slaughtered trade cattle in Maiduguri, Nigeria. JVMAH 8(8), 83–88. Rakwong, P., Keawchana, N., Ngasaman, R. and Kamyingkird, K. 2022. Theileria infection in bullfighting cattle in Thailand. Vet. World. 15(12), 2917. Saleh, M.A. 2009. Erythrocytic oxidative damage in crossbred cattle naturally infected with Babesia bigemina. Res. Vet. Sci. 86(1), 43–48. Sarasombath, P.T., Phuakrod, A., Taweechue, K., Loimek, S., Foongladda, S., Sermsart, B. and Wongkamchai, S. 2019. Molecular identification of tick-borne microorganisms and seasonal pattern of tick load on cattle in northern and southern provinces of Thailand. Southeast Asian J. Trop. Med. Public Health 50(6), 996–1005. Shit, N., Hajra, D.K., Mandal, M. and Mukherjee, R.D. 2023. Seasonal influence on prevalence of haemoprotozoan parasitic diseases in crossbred cattle under Terai-Dooars region of West Bengal, India. Explor. Anim. Med. Res. 13(2), 191–197. Sontigun, N., Boonhoh, W., Phetcharat, Y. and Wongtawan, T. 2022. First study on molecular detection of hemopathogens in tabanid flies (Diptera: Tabanidae) and cattle in Southern Thailand. Vet. World. 15(8), 2089. Srionrod, N., Nooroong, P., Poolsawat, N., Minsakorn, S., Watthanadirek, A., Junsiri, W., Sangchuai, S., Chawengkirttikul, R. and Anuracpreeda, P. 2022. Molecular characterization and genetic diversity of Babesia bovis and Babesia bigemina of cattle in Thailand. Front. Cell Infect. Microbiol. 12, 1065963. Strydom, T., Lavan, R.P., Torres, S. and Heaney, K. 2023. The economic impact of parasitism from nematodes, trematodes and ticks on beef cattle production. Animals 13(10), 1599. Subapriya, S., Senthil, N.R., Gowri, B., Chandrasekaran, D., Gopalakrishnan, A., Arunaman, C.S., Selvaraj, P. and Vairamuthu, S. 2021. Prevalence of haemoprotozoal diseases in cattle: a review of 6000 cases. Pharm. Innov. J. 10(8), 535–541. Swai, E.S., Karimuribo, E.D., Kambarage, D.M. and Moshy, W.E. 2009. A longitudinal study on morbidity and mortality in youngstock smallholder dairy cattle with special reference to tick borne infections in Tanga region, Tanzania. Vet. Parasitol. 160(1–2), 34–42. Tan, L.P., Hamdan, R.H., Hassan, B.N.H., Reduan, M.F.H., Okene, I.A.A., Loong, S.K., Khoo, J.J., Samsuddin, A.S. and Lee, S.H. 2021. Rhipicephalus tick: a contextual review for Southeast Asia. Pathogens 10(7), 821. Tefi, I.K., Satrija, F. and Cahyaningsih, U. 2015. Study the existence of blood parasites (Anaplasma, Babesia, Theileria) and physiological profiles of Australian imported feeder cattle. Acta Parasitol. Glob. 6, 55–59. Velusamy, R., Rani, N., Ponnudurai, G., Harikrishnan, T.J., Anna, T., Arunachalam, K., Senthilvel, K. and Anbarasi, P. 2014. Influence of season, age and breed on prevalence of haemoprotozoan diseases in cattle of Tamil Nadu, India. Vet. World. 7(8), 574–578. Weerasooriya, G., Sivakumar, T., Lan, D.T.B, Long, P.T., Takemae, H., Igarashi, I., Inoue, N. and Yokoyama, N. 2016. Epidemiology of bovine hemoprotozoa parasites in cattle and water buffalo in Vietnam. J. Vet. Med. Sci. 78(8), 1361–1367. Weny, G., Okwee-Acai, J., Okech, S.G., Tumwine, G., Ndyanabo, S., Abigaba, S. and Goldberg, T.L. 2017. Prevalence and risk factors associated with hemoparasites in cattle and goats at the edge of Kibale National Park, Western Uganda. J. Parasitol. 103(1), 16–33. Yéo, N., Gragnon, B.G. and Karamoko, Y. 2020. Hémoparasites Chez Les Ruminants Domestiques Dans Les Départements De Korhogo Et Sinématiali En Côte d’Ivoire. ESJ 16, 183. Yitayew, D. and Samuel, D. 2015. Tick borne hemoparasitic diseases of ruminants: a review. Adv. Biol. Res. 9(4), 210–224. | ||
| How to Cite this Article |
| Pubmed Style Bohman W, Chooruang N, Sakuna K, Jarujareet W, Areekit K, Chantip D. Prevalence of haemoparasitic infections and influencing factors among fighting bulls in Southern Thailand: A retrospective analysis. Open Vet. J.. 2024; 14(10): 2587-2598. doi:10.5455/OVJ.2024.v14.i10.8 Web Style Bohman W, Chooruang N, Sakuna K, Jarujareet W, Areekit K, Chantip D. Prevalence of haemoparasitic infections and influencing factors among fighting bulls in Southern Thailand: A retrospective analysis. https://www.openveterinaryjournal.com/?mno=205789 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i10.8 AMA (American Medical Association) Style Bohman W, Chooruang N, Sakuna K, Jarujareet W, Areekit K, Chantip D. Prevalence of haemoparasitic infections and influencing factors among fighting bulls in Southern Thailand: A retrospective analysis. Open Vet. J.. 2024; 14(10): 2587-2598. doi:10.5455/OVJ.2024.v14.i10.8 Vancouver/ICMJE Style Bohman W, Chooruang N, Sakuna K, Jarujareet W, Areekit K, Chantip D. Prevalence of haemoparasitic infections and influencing factors among fighting bulls in Southern Thailand: A retrospective analysis. Open Vet. J.. (2024), [cited January 25, 2026]; 14(10): 2587-2598. doi:10.5455/OVJ.2024.v14.i10.8 Harvard Style Bohman, W., Chooruang, . N., Sakuna, . K., Jarujareet, . W., Areekit, . K. & Chantip, . D. (2024) Prevalence of haemoparasitic infections and influencing factors among fighting bulls in Southern Thailand: A retrospective analysis. Open Vet. J., 14 (10), 2587-2598. doi:10.5455/OVJ.2024.v14.i10.8 Turabian Style Bohman, Wiruntita, Nantaporn Chooruang, Kitikarn Sakuna, Wipaporn Jarujareet, Kosit Areekit, and Dhiravit Chantip. 2024. Prevalence of haemoparasitic infections and influencing factors among fighting bulls in Southern Thailand: A retrospective analysis. Open Veterinary Journal, 14 (10), 2587-2598. doi:10.5455/OVJ.2024.v14.i10.8 Chicago Style Bohman, Wiruntita, Nantaporn Chooruang, Kitikarn Sakuna, Wipaporn Jarujareet, Kosit Areekit, and Dhiravit Chantip. "Prevalence of haemoparasitic infections and influencing factors among fighting bulls in Southern Thailand: A retrospective analysis." Open Veterinary Journal 14 (2024), 2587-2598. doi:10.5455/OVJ.2024.v14.i10.8 MLA (The Modern Language Association) Style Bohman, Wiruntita, Nantaporn Chooruang, Kitikarn Sakuna, Wipaporn Jarujareet, Kosit Areekit, and Dhiravit Chantip. "Prevalence of haemoparasitic infections and influencing factors among fighting bulls in Southern Thailand: A retrospective analysis." Open Veterinary Journal 14.10 (2024), 2587-2598. Print. doi:10.5455/OVJ.2024.v14.i10.8 APA (American Psychological Association) Style Bohman, W., Chooruang, . N., Sakuna, . K., Jarujareet, . W., Areekit, . K. & Chantip, . D. (2024) Prevalence of haemoparasitic infections and influencing factors among fighting bulls in Southern Thailand: A retrospective analysis. Open Veterinary Journal, 14 (10), 2587-2598. doi:10.5455/OVJ.2024.v14.i10.8 |