| Research Article | ||
Open Vet. J.. 2024; 14(10): 2642-2650 Open Veterinary Journal, (2024), Vol. 14(10): 2642–2650 Research Article Phytochemicals, antioxidant, and antimicrobial activities of Opuntia stricta fruits peelWissal Affi1, Abdalla A. Mohamed2,3, Neji Gharsallah4, Iryna Smetanska5 and Lazhar Zourgui1*1Research Laboratory BMA “Biodiversity, Molecules, Application” Higher Institute of Applied Biology of Medenine, University of Gabes, Medenine, Tunisia 2Department of Life Science, Faculty of Science, Laboratory of Plant Biotechnology Applied to Crop Improvement, University of Sfax, Sfax, Tunisia 3Department of Medical Nutrition, Faculty of Medical Technology, Biomedical Research Team, University of Zawia, Zawia, Libya 4Laboratory of Microbiology, Faculty of Science, University of Sfax, Sfax, Tunisia 5Plant Production and Processing, University of Applied Sciences Weihenstephan-Triesdorf, Freising, Germany *Corresponding Author: Lazhar Zourgui. Research Laboratory BMA “Biodiversity, Molecules, Application” Higher Institute of Applied Biology of Medenine, University of Gabes, Medenine, Tunisia. Email: lazhar.zourgui [at] gmail.com Submitted: 23/07/2024 Accepted: 08/09/2024 Published: 31/10/2024 © 2024 Open Veterinary Journal
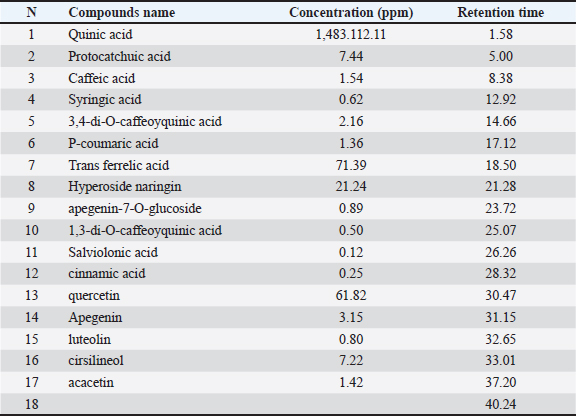
AbstractBackground: Currently, research is focused on therapeutic plants which are regarded as a supply of several phytotherapeutic compounds with several activities; among these plants we find cacti. Aim: The purpose of this work is to reveal the phytochemical composition and the antioxidant and antimicrobial properties of fruit peel from Opuntia stricta. Methods: The phenolics profile has been obtained by liquid chromatography/electrospray ionization/mass spectroscopy analysis, antioxidant capacity has been evaluated by DPPH, FRAP, CAT, and ABTS• + free radical, and the antimicrobial activity was tested against 7 bacteria and 3 fungi. Results: The results reveal that EFOS has a high content of polyphenols, flavonoids, quinic acid, and hyperoside which were the most dominant. Moreover, the observed antioxidant capacity of the extract was remarkable. In addition, a high antimicrobial capacity especially against Staphylococcus aureus and Pythium catenulatum. These results indicate that fruit peels of O. stricta have potential applications as a natural preservative in cosmetics and in food formulations, and also as a natural remedy. Conclusion: The finding proved that EFOS is characterized first by a wide variability of polyphenols and flavonoids and secondly by good potential antioxidant and antibacterial activities. Keywords: Opuntia stricta, Phytochemical composition, LC-ESI-MS analysis, Antioxidant, Antimicrobial activities. IntroductionFor many centuries man has continued to seek and support himself by tapping into nature, which not only provides him with his nutritional and clothing needs but also drugs. Even today, human tradition has developed the knowledge and the use of medicinal plants to improve human health (Iserin, 2001). Two-thirds of the pharmacopeia still use the curative properties of plants because the effectiveness of the drugs decreases in view of their side effects on public health. These health-promoting plants have the potential to be considered as an inexhaustible origin of secondary metabolites and organic compounds with structural diversity that otherwise would not be available in a chemical synthesis laboratory (Koehn and Carter, 2005). Opuntia (cactus) is an important medicinal plant that is native to Mexico (Orwa et al., 2009). Its geographical distribution is very wide; it was first introduced in Spain and then in South and North Africa (Arba, 2009), and due to the hot, arid, and semi-arid climate necessary for its development, Tunisia is one of the countries with the largest cactus growing areas in the world. The genus Opuntia includes many species that have certainly aroused the interest of biologists and phytochemists. Opuntia stricta is a mythical plant that grows easily in Tunisian soil and climate conditions. It is already seen as the second most common cactus after Opuntia ficus indica. Opuntia stricta presented a potential interest in medicine and human health. With all its parts, it belongs to the plants, which are the most used in traditional medicine for their beneficial effects. Recent studies have shown that O. stricta offers various benefits such as: antidiabetic effects observed in cladodes, which can decrease blood levels (Kunyanga et al., 2014). Anti-inflammatory effects against RAW 264.7 cells as well as the antioxidant and cytotoxic effects were found in aqueous extracts, ethanol, and acetone of O. stricta cladodes (Izuegbuna et al., 2019). Another study used DPPH radical analysis to show that O. stricta fruit peel has a significant antioxidant effect (Koubaa et al., 2015). Many medical researchers are rediscovering this genus and their richness in bioactive molecules. Opuntia stricta cladodes have taken attention for its richness in vitamins (vitamin A, vitamin C, and vitamin E), fibers, essential oils, and some phytochemicals (Díaz et al., 2017; Izuegbuna et al., 2019). Studies have shown their richness in calcium than vegetables and fruits. Further research has shown that O. stricta fruit includes protein, calcium, magnesium, zinc, sodium, and vitamin (Lim, 2012). According to the study of group Kunyanga et al. (2014), cactus pulp also contains ascorbic acid, phosphate, glucose, and fructose. Another study found that Opuntia fruit juice was a possible source of beta-cyanine pigments and could be used as a natural red-purple food colorant. The work carried out by Kunyanga et al. (2014) shows that seeds contain higher concentrations of proteins, oil, fiber, and carotenoids than pulp. As the interest of the various parties could come from its bioactive molecules, we were interested in fruit peels with a view to their possible valorization for biomedical use. Despite the fact that peels represent 2/3 of the total fruit (Koubaa et al., 2015) and are characterized by their richness in bioactive compounds (Amaya et al., 2019), they are generally not consumed by humans and have been largely neglected. It is with this objective that our research was included by having a number of means and analysis techniques to explore the bioactive potential of this mythical plant. To achieve this objective, the present research study has been intended to evaluate the content of polyphenols, flavonoids, and tannins, as well as to confirm this composition by liquid chromatography/electrospray ionization/mass spectroscopy (LC-ESI-MS). And then, to determine the antioxidant and antimicrobial properties of O. stricta peel extract. Materials and MethodsChemical materialChemical reagents namely Mueller Hinton Agar have been obtained from Bio-Rad France. MTT, Folin–Ciocalteu reagent, DPPH, BHT, DMSO, TCA, ABTS, AlCl3, FeCl3, Ethanol, gallic acid, catechin, ascorbic acid, linoleic acid, vanillin, and thiobarbutiric acid, have been acquired from Sigma, France. Everything else in chemical materials has been of the analytical variety. Plant materialMature fruits of O. stricta were harvested on February 2020, from Tunisia (Sidi Bouzid: Latitude: 35° 02′17″ North, Longitude: 9 29′05″, Altitude: 332 m). This plant was identified by Pr. Mohamed Echaib, Professor at the Faculty of Sciences, Sfax, Tunisia. Each fruit was peeled to separate its peel from the pulp after being cleaned with distilled water. Moreover, fruit peel was sliced in fine sections and dried in a drying oven at a temperature of 50°C for 18 hours, moreover, crushed using an apparatus Nima electric shredder (nima®, Japan). They are finally preserved in closed containers until their use for the extraction of the rough extracts. Culture and conservation of microorganismsAntimicrobial activity was tested for seven bacterial strains, comprising: Bacillus subtilis (JN 934392), Bacillus cereus (JN 934390), Staphylococcus aureus (ATCC 6538), Micrococcus luteus, Salmonella enteritidis (ATCC43972), Escherichia coli (ATCC 25922), and Klebsiella pneumonia. And against three fungal strains: Fusarium oxysporum (AB586994), Pythium catenulatum (AY598675), and Fusarium sp (JX391934). Preparation of extract200 g powder of the fruit peel had been macerated in 800 ml ethanol during 24 hours under agitation. The mixture was centrifuged at 4,500 rpm during 15 minutes. Then, the ethanol is separated by evaporation using a rotary evaporator. The pellet was kept at 4°C. Determination of the phytochemical composition of the peel extractTotal phenolics The total phenol content was analyzed using the reagent of Folin-Ciocalteu (Dewanto et al., 2002). The intensity of the blue color informs about the quantity of total polyphenols in the extract. By mixing 125 μl of each extract with 125 μl of the Folin-Ciocalteu reagent in 500 μl of distilled water. After stirring and resting for 3 minutes, 1,250 μl of a sodium carbonate solution (7% CO3Na2) is placed in the presence of the reaction medium in distilled water to a volume of 3 ml. The solutions are then exposed to darkness for 90 minutes at room temperature. Using a spectrophotometer, their absorbance (at 760 nm) was measured in relation to a blank. Under the same conditions, a standard range based on gallic acid is prepared in parallel to measure total polyphenols. Total polyphenol concentrations are measured in mg of gallic acid equivalent per g dry matter (mg AGF/g DM). Each sample is examined three times. Flavonoids content The flavonoid content was determined using the colorimetric technique as explained by Dewanto et al. (2002). This process is predicated on the formation of a complex between the flavonoid and the aluminum chloride (AlCl3). A 250 μl sample of each extract is mixed with 75 μl of NaNO2 solution (5%). After 6 minutes of incubation at room temperature, 150 μl of a solution of (ALCL3, 6H2O) is added to the mixture. After incubation for 5 minutes at room temperature, 500 μl of NaOH (1M) is added to the mixture and then corrected to 2,500 μl with distilled water. The absorbance of the different solutions at 510 nm is then evaluated using a spectrophotometer. A catechin-based calibration curve expresses the total amount of flavonoids in equivalent milligrams of catechin per gram of dry matter (mg EC/g MS). Each sample is examined three times. Measurement of tannin content Tannins content was evaluated by the vanillin approach described by Belhaj et al. (2016). This compound depolymerizes in the presence of concentrated sulphuric acid and converts into anthocyanidols when vanillin is present. 50 μl of each extract is added to 3 ml of vanillin solution (4%) and 1,500 μl of pure hydrochloric acid. The mixture is then incubated for 15 minutes at room temperature and the absorbance is evaluated by spectrophotometry at 500 nm. The color changing to a greenish brown indicates the presence of tannins. Using a catechin-based calibration curve, the amount of condensed tannins can be measured by using one equivalent milligram of catechin per gram of dry matter (mg EC/g MS). Each sample is examined three times. Analysis of LC/ESI/MS The chemical composition of EFOS was evaluated by LC-ESI-MS according to the method described by Ayaz et al. (2005). The analysis was performed on a quadripolar mass spectrometer LC-MS 2020 (Shimadzu, Kyoto, Japan) using an online liquid chromatography system equipped with an LC-20AD XR binary pump system, a column furnace CTO-20AC, an automatic sampler SIL-20AC XR, and a degreaser DGU-20A 3R (Shimadzu). The chromatographic separation was performed using AQUASIL C18 analytical column (150 mm, 3 mm, and 3 μm) from Thermo Electron, Dreieich, Germany was used for the chromatographic separation, followed by an Aquasil C18 protective column (10 mm, 3 mm, 3 μm, Thermo Electron). Two solvents: solvent A (0.1% formic acid in H2O, v/v), and solvent B (0.1% formic acid in methanol, v/v) with linear gradient elution and a 5 minutes rebalancing interval in between each run, make up the mobile phase. This mobile phase’s flow rate was kept constant at 40°C at 0.4 ml/minute. Next, the Shimadzu Lab Solutions LC-ESI-MS software was used to process the spectra, using an injection of a volume of 5 μl. The full scan spectra from 50 to 2,000 Da, a voltage detector of 1.2 V, block source and dissolution line temperatures of 400°C and 250°C, dry gas flow rates of 12 l/minute and 1.5 l/minute, respectively; were all part of the negative ionization mode mass spectra data. Analysis of the antioxidant capacity of the peel extractDeterminations of anti-radical activity of O. stricta extract using the DPPH method The DPPH method is the most rapid test. It was realized based on the method reported by Yahyaoui et al. (2018) and based on the appearance of a discoloration. This discoloration explains the capacity of EFOS to trap this radical. A volume of 100 µl of an ethanolic extract at different levels of concentrations (from 0.012 to 5 mg/ml) was incorporated into 1.9 ml of ethanolic solution of DPPH (0.025 g/l=2.5 mg/100 ml ethanol). Concurrently, a control is made by combining 100 µl ethanol and 1. 9 ml of the ethanolic DPPH solution, a trolox and ascorbic acid solution serves as a positive control. After 30 minutes of incubation, the absorbance at 515 nm is measured. The results were represented by the percentage of inhibition (PI %) calculated using the following formula: PI (%)=(At- Ae)/At × 100 At: Total absorbance (100%) Ae: Absorbance of extract solution These results provided an opportunity to measure the percentage of PI (%) as a function of concentration, which allowed the determination of the inhibitory concentration IC50. Determination of the antioxidant capacity of O. stricta extract using the FRAP method FRAP method is a universal assay. It can be assessed using the technique outlined by Cheurfa and Allem (2016). This method is based on the existence of the reducers in EFOS which results in the decrease of iron Fe3+ complex ferricyanide with ferrous form. In order to make the functioning FRAP reagent, 1 ml of extract at different concentrations was combined with 2.5 ml of the plug phosphates (pH=6.6; 0.2 M) and potassium ferricyanide [K3Fe (CN) 6] (1%) (2.5 ml). Moreover, mixtures were kept in a water bath at 50°C for 20 minutes. Following the incubation period, the aliquot ones a trichloracetic acid (TCA: 2.5 ml) were introduced to the combinations, after underwent centrifugation during 10 minutes at 4,500 rpm. A combination of 0.5 ml of FeCl3 and 2.5 ml of pure water are added with the recovered supernatant. After 10 minutes, the absorbance is measured at 700 nm. The extract is replaced by an extraction solvent to obtain a prepared blank. The positive control test used was a solution of a conventional antioxidant whose absorbance was evaluated under the same conditions as the observed samples. By increasing the absorbance, an increase in the reducing power of the extracts tested is observed. The reducing power was measured in milligrams of extract per milliliter using a calibration curve. Test of antioxidant capacity by phosphomolybdate The total antioxidant activity (TAC) had been evaluated utilizing the phosphomolybdene technique outlined by Prieto et al. (1999). 28 mM sodium phosphate, 4 mM ammonium molybdate, and 0.6 M sulphuric acid were blended. And, 3 ml of this solution is combined with 0.3 ml of EFOS. After that and for 90 minutes, the sample is kept at 95°C. Following incubation and chilling, we measured the absorbance at 695 nm of the solutions and the control that contained the ethanol (0.3 ml) and the reagent solution (3 ml). Finally, the amount of ascorbic acid equivalent milligrams per gram of dry matter represents the total antioxidant potential. The anti-radical activity of O. stricta extract by the ABTS test The study of the antioxidant activity was performed using the method TEAC (Chang et al., 2008). The TAC of a molecule was deduced from its capacity to inhibit the radical cation ABTS•+, obtained starting from the ABTS compared to Trolox. The absorbance was measured at 734 nm. The fading extinction was calculated as a function of the percentage decrease in absorbance. This percentage was calculated based on the concentration and compared to the equivalent Trolox concentration. Thus, activity was measured in mg of Trolox equivalent per g sample. Antimicrobial screeningPreparation of the microbial suspensions The test bacteria were cultivated on an agar Mueller Hinton (MH) in Petri dishes and incubated for 24 hours at 37°C at 200 rpm of agitation. The OD read at 625 nm was justified with 0.08–0.10 density about 106 UCF/ml (Daoud et al., 2016). As for the fungi stocks, at 30°C the growth of the isolates was performed; until the mycelia development completely covered all dishes on agar Sabouraud. Into these dishes, the spore solution is made in 10 ml of water with sterile tween. Likewise, the OD is changed to between 0.08 and 0.10 to produce a solution that contains 106 spores per milliliter (Bakari et al., 2018). Measurement of antimicrobial propriety by agar technique This technique consists making a cavity of 6 mm diameter in solidified agar MH. In fact, the agar Muller Hinton (20 ml) was placed in a petri dish and after solidification, 100 μl of the suspension of bacteria /solution of spores to be examined was distributed across the surface of the agar using a sterile flue brush. In order to, 60 μl of every extract (150 mg/ml DMSO) was added to every well. A negative test was conducted concurrently. The dish was incubated for 24 hours at 37°C and for 4–7 days at 30°C for fungi and bacteria (Daoud et al., 2016). The results were expressed in mm and the tests were repeated three times. The sensitivity was classified according to the diameter of the inhibition halo. Measurement of MIC and minimum fungicidal concentration (MFC) using the micro-dilution well techniqueBacterial and fungus stocks demonstrating awareness to the extract tested were chosen to assess MIC, MBC, and MFC. The MIC was given based on the procedure outlined by Daoud et al. (2016) in the sterile plate ELISA (contains 96 wells). The extract was dissolved in the DMSO. Different dilutions were made to get a variety of concentrations ranging from 150 mg to 1.10. Therefore, the agar MH (90 μl) for bacteria and fungus respectively, were scattered in every well while incorporating 10 μl of every inoculum previously prepped, and 100 μl of every dilution of the extract was thus poured. Following the content homogenization, the plates are incubated for 24 hours at 37°C to grow the bacterial stock and with 30°C during 3 days for the fungus stocks. Adding 25 μl MTT in every well and then incubating the mixture for 30 minutes at 37°C. On the one hand, wells or microbial growth is inhibited and stays transparent during MTT incubation (Hsouna et al., 2011). On the other hand, minimum bactericidal concentration (MBC) inhibits all the growth visible of the micro-organisms during 48 hours at 37°C. For fungus stocks, upon incubation, the initial tubes with inhibition that is total, in comparison with witnesses, kept at 30°C during 3 to 4 days, are planted in a plate with Sabouraud without of extract. The amount is referred to as the MFC. An examination of statisticsThe experiment outcomes data were run through three replicates and reported as mean ± SEM. One-way analysis of variance was used to evaluate differences, and the least significant difference (LSD) test was used to track changes and determine the significance of the main effects at the 5% probability level. Ethical approvalNot needed for this study. ResultsPhytochemicals compositionIn view of the richness of the cactus in secondary metabolites, we sought to determine total phenolic content, flavonoids content, and tannins content in EFOS using specific standard curves (gallic acid and catechin). Table 1 shows that there is a high level of polyphenols (56 ± 0.06 mg GAE/g dry matter), flavonoids (24.6 ± 0.45 mg CE/g of dry matter) and condensed tannins (5.09 ± 0.07 mg CE/g dry matter). LC-ESI-MS analysisThe highest content of total polyphenols and flavonoids compounds contains in EFOS required to be confirmed by LC-ESI- MS analysis. In fact, the current research is the first to qualify and quantify phenolic compounds of EFOS from the center of Tunisia. In this investigation, we used LC-ESI- MS that enabled us to identify 16 compounds (Quinic acid, protocatchuic acid, naringin, hyperoside, caffeic acid, syringic acid, p-coumaric acid, trans ferrelic acid, 1,3-di-O-caffeoyquinic acid, salviolonic acid, quercetin, cinnamic acid, , apegenin, luteolin, cirsilineol, acacetin) summarized in Table 2 and expressed as ppm of content. The most abundant compounds were quinic acid and hyperoside with 1,483.19 and 71.39 ppm respectively. Table 1. The polyphenols, flavonoids and condensed tannins content in the ethanolic extract of fruits peel of O. stricta.
Table 2. Chemical composition in the ethanolic peels extract of O. stricta by LC-ESI-MS.
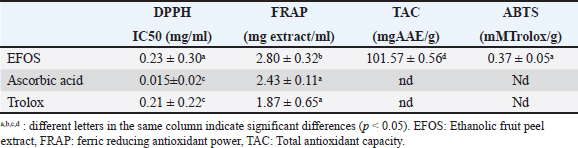
Table 3. Antioxidant profile of peel from O. stricta extract and standards compounds.
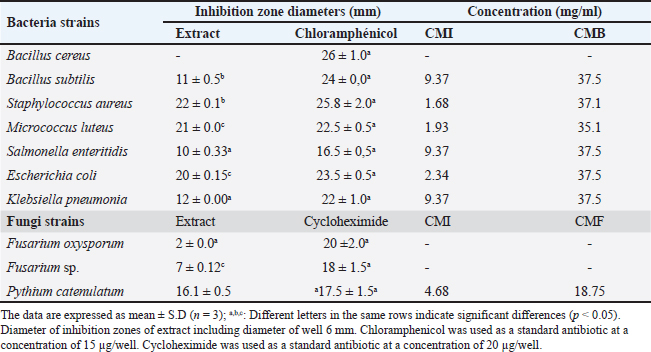
Evaluation of antioxidant capacityThe extract’s antioxidant capacity and bioactive molecules have been estimated by different assays. First, the anti-radical activity of O. stricta extract was detected by DPPH. Our results were expressed in IC50 and compared to trolox. In addition, these results show that the extract of the peel had IC50 around 0.23 ± 0.30 mg/ml. So, it has an antioxidant power close than trolox with 0.21 ± 0.22 mg/ml (Table 3). Second, FRAP was utilized to assess the reducing power of EFOS, with ascorbic acid serving as the reference material. According to Table 3, the maximum absorbance for the extract at a concentration of 1 mg/ml was approximately 2.80, whereas for the ascorbic acid at a concentration of 0.4 mg/ml, it was 2.43. In conclusion, EFOS had a high total antioxidant capacity, meanwhile, the Scavenging activity of ABTS• + free radical presented in Table 3 is about 0. 37 ± 0.05 mmol of Trolox/g of extract. Our sample inhibited the absorption of the ABTS+• radical and this inhibition was dose-dependent (the dose-dependent effect was observed). Evaluation of antimicrobial capacitySeven bacteria and three fungi were used to test the antimicrobial propriety of EFOS. The activities of these strains were analyzed both qualitatively and quantitatively using the minimum inhibitory concentrations (MICs), the minimum bactericidal concentrations (BMCs), and the MFCs, and diameters of inhibition zones. The findings attained are then compared with chloramphenicol and cycloheximide. In fact, the antibacterial activity test of fruits peel is performed by the agar diffusion method. Then, the obtained result shows that EFOS showed significant antibacterial efficacy against a wide variety of microbes. Among all bacteria, S. aureus, M. luteus, are the most sensitive with inhibition diameter values (22; 21 mm), respectively. And, the most susceptible strain is reported to be S. aureus. Regarding antifungal activity, the peel has areas of inhibition against the fungal strains tested. According to Table 4, EFOS has a mean inhibition zone against P. catenulatum (16.1 ± 0.5 mm) and no activity was against the Fusarium oxysporum and Fusarium sp. Furthermore, to better study antimicrobial activity MIC, BMC, and FMC have been determined. The outcomes obtained for the various microorganisms examined in terms of MIC, MBC, and MFC agree with the diameters of the inhibitory zones. The study revealed relatively high MIC, MBC, and MFC. Values for the extract studied, indicate moderate antibacterial potency. The results obtained in our experiments show that the EFOS has a bactericidal effect against the growth of most of the strains tested. DiscussionSince antiquity, plants have been part of human daily life, as they use them to feed and heal (Fellah et al., 2006). Furthermore, it is currently demonstrated that 20% of types of plants that are now in cultivation around the world have benefits for health or beauty because they contain molecules or active substances with a variety of biological characteristics (Suffredini et al., 2004). In this context, the fruit peel of O. stricta collected from Sidi Bouzid (Tunisia) was studied for its bioactive compounds, antioxidant and antimicrobial capacities. In fact, this work highlights a very important phytochemical composition. Indeed, the highest content of polyphenols and flavonoids has been shown in fruit peels. These amounts are in discordance with many studies: The study conducted by Anaberta et al. (2010) reported that EFOS from Villa de Graciano, San Luis Potosí, Mexico having a low PC, FC, and TC (3.62 mg GAE/g of dry mater, 0.66 mg CE/g of dry mater, together with 0.53 mg CE/g of dry mater respectively). Also, Ben Lataief et al. (2020) reported a few contents of PC, FC and TC from Opuntia dillenii fruit peel extract (PC: 18.40 ± 0.22 mg GAE / g; FC: 7.13 ± 0.01 mg CE /g). Moreover, Zourgui et al. (2020) demonstrate that the concentration of phenolic compounds: polyphenols (24.65 ± 0.5 mg GAE/ g) and flavonoids (14.08 ± 0.03 mg CE/ g) in the ethanolic extract Opuntia streptacantha fruit peel. Then, Aruwa et al. (2019) proved a low PC (17.59 ± 0.02 mg GAE/g) and FC (14.83 ± 0.10 mg GAE/g) in O. ficus indica fruit showed. This implies that the ethanolic extract of EFOS contained more phenolic compounds than the other species of Opuntia (O. dillenii, O. streptacantha, and O. ficus indica). This difference between the Opuntia species may be associated with weather, localization, nature of the soil, plant age… Table 4. Antimicrobial activities, MIC, MBC, and MFC (mg/ml) of cactus peel extract against bacterial and fungal strains.
The increased levels of phenolic compounds were affirmed and detected by LC-ESI-MS analysis which showed the presence of 9 polyphenols and 7 flavonoids with the dominance of quinic acid and hyperoside. In literature, several types of research indicated that phenolic acids and flavonoids exhibited various biological properties (Santos et al., 2016). Zanello et al. (2015) demonstrated that quinic acid functions as a natural protector against cancer, the virus and oxidative stress. Also, Bakari et al. (2018) signaled that hyperoside is characterized by antibacterial along with anti-inflammatory and antioxidant properties. Comparing this study to other work, it was found that EFOS has a higher level in quinic acid and hyperoside than ethanolic extract of the same extract from O. streptacantha which contains (17.54 ppm of quinic acid and 35.11 ppm of hyperoside) (Zourgui et al., 2020). Similarly, the ethanolic extract of fruit peel from O. dillenii has a low concentration of Quinic acid, cinnamic acid, and hyperoside with 1,437.03, 40.19, and 0.92 ppm, respectively (Ben Lataief et al., 2020). Interestingly, this richness in total phenolic content devotes our extract to an interesting antioxidant activity. To be better study of O. stricta, an assessment of antioxidant and antimicrobial activity has been carried out. The results showed a higher level of antioxidant capacity than the extract from O. stricta peel from the middle of Tunisia, region of Al-Ala, and O. ficus indica (0.40 ± 0.03 and 0.57 ± 0.02, respectively) (Yeddes et al., 2013). Furthermore, the finding demonstrated that when sample concentrations increased, the ferric reducing increased. The present discovery is comparable to those reported by Ben Lataief et al. (2020). At any given FRAP concentration in extract from O. dillenii peels is 1.45 ± 0.03 mg/ml. We can draw the conclusion that EFOS is a more potent antioxidant. Olelaye and Rocha’s (2007) finding, which demonstrates that the high content of phenolic compounds in cactus peel is related to its high antioxidant capacity antioxidant activity, indicates that the richness in polyphenols and flavonoids in the peel may be linked to its antioxidant activity. Cacti polyphenols are known to provide significant protection against oxidative aggression. Concerning the antimicrobial propriety monitored in vitro, it was demonstrated a better antimicrobial activity for fruit peel especially against S. aureus. In fact, the present research proved that the antibacterial activities were classified as the Gram+ bacteria the more sensitive than the gram- bacteria. This observation is probably related to the nature of the Gram+ bacteria cell wall, which is characterized by peptidoglycan that facilitates the penetration of aldehydes or phenols contained in the ethanolic extract of fruit peel from Opuntia stricta. Contrarily the Gram- bacteria are characterized by lipopolysaccharide that restricts the transport through the cell wall (Koubaa et al., 2015). Our findings align with existing literature regarding EFOS’s antimicrobial efficaciousness. To make clear the activity of the different strains, additional research was conducted using the micro-dilution method to determine MIC, MBC, and MFC of EFOS against various fungal and bacterial strains (Daoud et al., 2016). The present discovery aligns with the outcomes acquired by Kharrat et al. (2018) who proved the good antimicrobial activity of O. stricta peel. Interestingly, other authors confirmed that the red prickly pear can reduce the inhibition zone of microorganisms (Gram+ or Gram-) (Ali and El-Mohamedy, 2011). Finally, we can conclude the efficacy of peel extract as a biological control agent in the growth of bacteria. Therefore, O. stricta can be used as a biological remedy to control these pathogens because of how much phenolic chemicals they contain. ConclusionEventually, it is the only research about the fruit peel of O. stricta from Tunisia center. The findings proved that EFOS is characterized first by a wide variability of polyphenols and flavonoids and secondly by a good potential antioxidant and antibacterial activity. Following the richness of fruit peel in phytochemical composition and on interesting biological activities. This work deserves to be supplemented, to deepen the research by other more exhaustive, more in-depth, and complementary techniques. AcknowledgmentsThe research project was supported by the Tunisian Ministry of Higher Education and Scientific research via the Research Laboratory BMA “Biodiversity, Molecules, Application” Higher Institute of Applied Biology of Medenine, University of Gabes. Conflict of interestThe authors have disclosed no conflicts of interest. FundingThis research received no external funding. Authors’ contributionsLZ drafted the manuscript. WA and AAM revise and edit the manuscripts. WA and NG took part in preparing and critically checking this manuscript. IS, LZ, and WA performed the methodology. All authors have read and agreed to the published version of the manuscript. Data availabilityData are available upon request from the corresponding author. ReferencesAli, N. and El-Mohamedy, R. 2011. Eco-friendly and protective natural dye from red prickly pear (Opuntia Lasiacantha Pfeiffer) plant. J. Saudi Chem.15, 257–261. Anaberta, C., Cristian, J. and Georgina, S. 2010. Revalorization of cactus pear (Opuntia spp.) wastes as a source of antioxidants. Ciencia. Tecnol. Alime. 31, 782–788. Amaya, D., Perez, I., Garcia, J., Jacobo, C., Espinoza, A. and Camacho, R. 2019. An integral profile of bioactive compounds and functional properties of prickly pear (Opuntia ficus indica L.) peel with different tonalities. Food Chem. 278, 568–578. Arba, M. 2009. Le cactus, Opuntia, une espèce fruitière et fourragère pour une agriculture durable au Maroc. In Symposium International Agriculture durable en region. Hassan II Agricultural and Veterinary Institute, Agadir, Morocco. Aruwa, C., Amoo, S. and Kudanga, T. 2019. Extractable and macromolecular antioxidants of Opuntiaficus-indica cladodes: phytochemical profiling, antioxidant and antibacterial activities. S. Afr. J. BOT 125, 402–410. Ayaz, F., Hayirlioglu-Ayaz, S., Gruz, J., Novak, O. and Strnad, M. 2005. Separation, characterization, and quantitation of phenolic acids in a little-known blueberry (Vacciniumarctostaphylos L.) fruit by HPLC-MS. J. Agric. Food Chem. 53, 8116–8122. https://doi.org/10.1021/jf058057y. Bakari, S., Hajlaoui, H., Daoud, A., Mighri, H., Garcia, J., Gharsallah, N. and Kadri, A. 2018. Phytochemicals, antioxidant and antimicrobial potentials and LC-MS analysis of hydroalcoholic extracts of leaves and flowers of Erodiumglaucophyllum collected from Tunisian Sahara. Food Sci. Technol. 38, 310–317. Belhaj, F., Somrani, I., Aissaoui, N., Messaoud, C., Boussaid, M. and Marzouki, N. 2016. Bioactive compounds contents, antioxidant and antimicrobial activities during ripening of Prunuspersica L. varieties from the North West of Tunisia. Food Chem. 204, 29–36. Ben Lataief, S., Zourgui, M., Rahmani, R., Affi, W., Daoud, A., Gharsallah, N. and Zourgui, L. 2020. Phytochemical composition and evaluation of antioxidant and antimicrobial activities of Tunisian Opuntia dellinii peel fruits. EJPMR 7, 149–160. Chang, S.F., Hsieh, C.L. and Yen, G.C. 2008. The protective effect of Opuntia dillenii Haw fruit against low-density lipoprotein peroxidation and its active compounds. Food Chem. 106, 569–575. Cheurfa, M. and Allem, R. 2016. Evaluation of antioxidant activity of different extracts of Aloysia triphylla leaves (L’Herit.) from Algeria in vitro. J. Photother. 14, 181–197. Daoud, A., Smaoui, S., Kadri, A. and Gharsallah, N. 2016. Proximate analysis, mineral composition, phytochemical contents, antioxidant and antimicrobial activities. Arab. J. Chem. 37(2), 234–287. Dewanto, V., Wu, X., Adom, K. and Liu, R. 2002. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 50, 3010–3014. Díaz, M.S.S., de La Rosa, A.P.B., Héliès-Toussaint, C., Guéraud F. and Nègre-Salvayre, A.J. 2017. Opuntia spp.: characterization and benefits in chronic diseases. Oxid. Med. Cell Longev. 2017, 8634249. Fellah, S., Romdhane, M. and Abderraba, M. 2006. Extraction et étude des huiles essentielles de la Salvia officinalis L. cueillie dans deux régions différentes de la Tunisie. J. Soc. Alger. Chim. 16(2), 193–202. Hsouna, A., Trigui, M., Mansour, R., Jarraya, R., Dammak, M. and Jaoua, S. 2011. Chemical composition, cytotoxicity effect and antimicrobial activity of Ceratoniasiliqua essential metal elements oil with preservative effects against Listeria inoculated in minced beef meat. Int. J. Food Microbiol. 148, 66–72. Iserin, P. 2001. Identification, préparation, soins. (Ed.). Larousse. Izuegbuna, O., Otunola, G. and Bradley, G.J. 2019. Chemical composition, antioxidant, anti-inflammatory, and cytotoxic activities of Opuntia stricta cladodes. PLoS One 14(1), 0209682. Kharrat, N., Salem, H., Mrabet, A., Aloui, F., Triki, S., Fendri, A. and Gargouri, Y. 2018. Synergistic effect of polysaccharides, betalain pigment and phenolic compounds of red prickly pear (Opuntiastricta) in the stabilization of salami. J. Biol. Macromol. 17, 31707–31715. Koehn, F. and Carter, G. 2005. The evolving role of natural products in drug discovery. Nature Rev. Drug Discov. 4, 206–220. Koubaa, M., Ktata, A., Barba, F., Grimi, N., Mhemdi, H., Bouaziz, F., Driss, D. and Elloz, S. 2015. Water-soluble polysaccharides from OpuntiastrictaHaw fruit peels: recovery, identification and evaluation of their antioxidant activities. Int. Agrophys. 29, 299–306. Kunyanga, C., Vellingiri, V. and Imungi, K.J. 2014. Agriculture, nutrition, development. Nutritional quality, phytochemical composition and health protective effects of an under-utilized prickly cactus fruit (Opuntia stricta Haw.) collected from Kenya. AJFAND 14(67), 9561–9577. Lim, T. 2012. Opuntia stricta. Edible medicinal and non-medicinal plants. Springer, Australia, pp: 687–692. Olelaye, M. and Rocha, B. 2007. Acetaminophen-induced liver damage in mice: effects of some medicinal plants on the oxidative defense system. Exp. Toxicol. Pathol. 59(5), 319–27. Orwa, C., Mutua, A., Kindt, R., Jamnadass, R. and Simons, A. 2009. Agroforestree database: a tree reference and selection guide version 4. Prieto, P., Inyeda, M. and Guilar, M. 1999. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem. 269, 337–341. Santos, U.P., Campos, J.F., Torquato, H.F., Paredes-Gamero, E.J., Carollo, C.A., Estevinho, L.M., de Picoli Souza, K. and Dos Santos, E.L. 2016. Antioxidant, antimicrobial and cytotoxic properties as well as the phenolic content of the extract from Hancornia speciosa Gomes. PLoS One 11(12), e0167531. Suffredini, J., Sader, H., Goncalves, A., Reis, A., Gales, A. and Varella, A. 2004. Screening of antimicrobial extracts from plants native to the Brazilian Amazon rainforest and Atlantic forest. Brazil. J. Med. Biol. Res. 37, 379–384. Yahyaoui, A., Khedher, O., Rigane, G., Ben Salem, R. and Moussaoui, Y. 2018. Photochemical analysis, antioxidant and antimicrobial activitiesof Eucalyptus essential oil: a comparative study between Eucalyptus marginata L. and Eucalyptus paucilora L. Rev. Roum. Chim. 64, 1055–1062. Yeddes, N., Jamila, K., Chérif, G., Guyot, S., Sotin, H. and Ayadi, T. 2013. Comparative study of antioxidant power, polyphenols, flavonoids and betacyanins of the peel and pulp of three tunisian Opuntia forms. J. Antioxid. 2, 37–51. Zanello, P., Koishi, A., Júnior, C., Oliveira, L., Pereira, A., Almeida, M.N. and Duarte, C. 2015. Quinic acid derivatives inhibit dengue virus replication in vitro. Virol. J. 12, 223–236. Zourgui, M., Hfaiedha, M., Brahmi, D., Affi, W., Gharsallah, N., Zourgui, L. and Amri, M. 2020. Phytochemical screening, antioxidant and antimicrobial activities of Opuntia Streptacantha fruit skin. J. Food Meas. Charact. 25, 198–288. | ||
| How to Cite this Article |
| Pubmed Style Affi W, Mohamed AA, Gharsallah N, Smetanska I, Zourgui L. Phytochemicals, antioxidant and antimicrobial activities of Opuntia stricta fruits peel. Open Vet. J.. 2024; 14(10): 2642-2650. doi:10.5455/OVJ.2024.v14.i10.14 Web Style Affi W, Mohamed AA, Gharsallah N, Smetanska I, Zourgui L. Phytochemicals, antioxidant and antimicrobial activities of Opuntia stricta fruits peel. https://www.openveterinaryjournal.com/?mno=207508 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i10.14 AMA (American Medical Association) Style Affi W, Mohamed AA, Gharsallah N, Smetanska I, Zourgui L. Phytochemicals, antioxidant and antimicrobial activities of Opuntia stricta fruits peel. Open Vet. J.. 2024; 14(10): 2642-2650. doi:10.5455/OVJ.2024.v14.i10.14 Vancouver/ICMJE Style Affi W, Mohamed AA, Gharsallah N, Smetanska I, Zourgui L. Phytochemicals, antioxidant and antimicrobial activities of Opuntia stricta fruits peel. Open Vet. J.. (2024), [cited January 25, 2026]; 14(10): 2642-2650. doi:10.5455/OVJ.2024.v14.i10.14 Harvard Style Affi, W., Mohamed, . A. A., Gharsallah, . N., Smetanska, . I. & Zourgui, . L. (2024) Phytochemicals, antioxidant and antimicrobial activities of Opuntia stricta fruits peel. Open Vet. J., 14 (10), 2642-2650. doi:10.5455/OVJ.2024.v14.i10.14 Turabian Style Affi, Wissal, Abdalla A. Mohamed, Neji Gharsallah, Iryna Smetanska, and Lazhar Zourgui. 2024. Phytochemicals, antioxidant and antimicrobial activities of Opuntia stricta fruits peel. Open Veterinary Journal, 14 (10), 2642-2650. doi:10.5455/OVJ.2024.v14.i10.14 Chicago Style Affi, Wissal, Abdalla A. Mohamed, Neji Gharsallah, Iryna Smetanska, and Lazhar Zourgui. "Phytochemicals, antioxidant and antimicrobial activities of Opuntia stricta fruits peel." Open Veterinary Journal 14 (2024), 2642-2650. doi:10.5455/OVJ.2024.v14.i10.14 MLA (The Modern Language Association) Style Affi, Wissal, Abdalla A. Mohamed, Neji Gharsallah, Iryna Smetanska, and Lazhar Zourgui. "Phytochemicals, antioxidant and antimicrobial activities of Opuntia stricta fruits peel." Open Veterinary Journal 14.10 (2024), 2642-2650. Print. doi:10.5455/OVJ.2024.v14.i10.14 APA (American Psychological Association) Style Affi, W., Mohamed, . A. A., Gharsallah, . N., Smetanska, . I. & Zourgui, . L. (2024) Phytochemicals, antioxidant and antimicrobial activities of Opuntia stricta fruits peel. Open Veterinary Journal, 14 (10), 2642-2650. doi:10.5455/OVJ.2024.v14.i10.14 |