| Review Article | ||
Open Vet. J.. 2024; 14(10): 2539-2550 Open Veterinary Journal, (2024), Vol. 14(10): 2539-2550 Review Article Acute phase proteins patterns as biomarkers in bacterial infection: Recent insightsAmer Al Ali1 and Wageh Sobhy Darwish2*1Department of Medical Laboratory Sciences, College of Applied Medical Sciences, University of Bisha, Bisha, Saudi Arabia 2Department of Food Hygiene, Safety and Technology, Faculty of Veterinary Medicine, Zagazig University, Zagazig City, Egypt *Corresponding Author: Wageh Sobhy Darwish. Department of Food Hygiene, Safety and Technology, Faculty of Veterinary Medicine, Zagazig University, Zagazig City, Egypt. Email: wagehdarwish [at] gmail.com Submitted: 03/08/2024 Accepted: 07/09/2024 Published: 31/10/2024 © 2024 Open Veterinary Journal
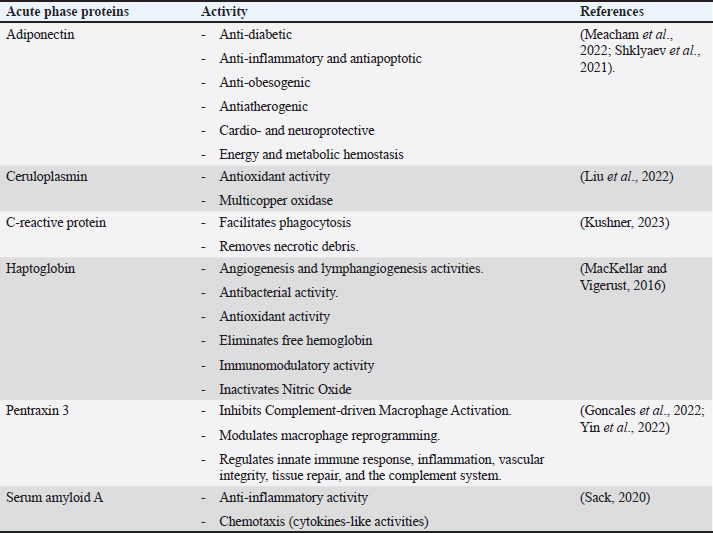
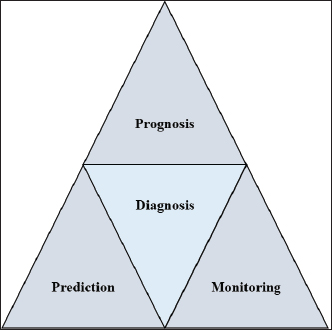
AbstractEscherichia coli is a bacterium with command and pathogenic variants. It has been implicated in the induction of several inflammatory conditions. Finding a biomarker for infection began many years ago. The challenge of using acute phase proteins (APPs) as biomarkers for infection is a promising target for many researchers in this field. Many APPs have been studied for their roles as biomarkers of E. coli infection. The following review aims to highlight recent trials that have approved the use of adiponectin, amyloid A, ceruloplasmin, C-reactive protein, Haptoglobin, and Pentraxin 3 as biomarkers for E. coli infection and assess the obtained results. In conclusion, despite the existing approaches for the use of APPs as biomarkers in E. coli infection, we recommend more precise studies to enable these markers to be more specific and applicable in clinical fields. APPs could be markers for systemic inflammatory conditions, regardless of the causative agent. Keywords: Adiponectin, Ceruloplasmin, C-reactive protein, Escherichia coli, Haptoglobin. IntroductionEscherichia coli is considered a paradigm for various bacterial species that have both commensal and pathogenic variants (Leimbach et al., 2013). It is classified as a rod-shaped, gram-negative bacterium belonging to the Enterobacteriaceae family. Although it can invade any organ, bacteria usually inhabit the guts of humans and other warm-blooded animals (Rumball et al., 2023). Distinguishing these species is difficult because of their instability, criteria, and character shifting (Cobo-Simon et al., 2023). The pathogenicity of E. coli depends on phenotypic traits and the expression of specific virulence factors (Sheikh and Fleckenstein, 2023). Escherichia coli is associated with many inflammatory conditions and diseases such as cholangitis (Zhang et al., 2022), Urinary tract infection (UTI) (Hashimoto et al., 2022), traveller’s diarrhoea (Muzembo et al., 2022; Yates, 2005), neonatal meningitis (Ku et al., 2015; Barichello et al., 2023), and pneumonia (Coe et al., 2022). This gives E. coli important value in the field of clinical research. Acute phase proteins (APPs) (Table 1) are early reactants in the majority of infectious diseases. They are involved in the acute phase response as part of innate immunity. APP patterns are altered in E. coli infection in many conditions and can be used to differentiate diarrhea caused by E. coli from other pathogens (Balikci and Al, 2014). The question here is whether this pattern change of APPs in serum and tissues of experimental models developed to be a potent marker for the diagnosis of E. coli infection. In addition, this pattern change could be used for early detection of infection. We focused on the recent involvement of APPs in E. coli infection and evaluated their role as diagnostic biomarkers or predictive parameters for E. coli infection. Are acute phase proteins considered biomarkers for E. coli infection?What is the biomarker? Table 2 shows APP patterns that have been studied for their use as biomarkers of E. coli infections. The definition and criteria of biomarkers were considered. Biomarkers are defined as biological substances that can be measured and provide an indication of physiological and pathological conditions or responses to exposures and interventions. As shown in Figure 1, Biomarkers should be specific for diagnosis and useful in monitoring, prediction, and prognosis of the disease (Kunc et al., 2020). Why are we seeking biochemical markers for bacterial infection? From our point of view, the biomarker used for detecting a bacterial infection should facilitate rapid diagnosis, predict infection patterns and prognosis, correlate with the consequences of the infection, and be used to monitor therapy and eradication. However, the question here is whether there is a biomarker specific to a unique bacterial infection. If there is, this will save time for multiple and complicated microbiological investigations of such bacteria, and it will save our time consumed in culturing. Currently, CRP and PCT levels can be routinely measured in clinics as biomarkers of bacterial infections. Others have directed the use of different markers to differentiate between bacterial and viral infections (He et al., 2022), despite there being no single biomarker for such differentiation to date. Biomarkers can also be used to differentiate between infectious and non-infectious inflammatory conditions (Zandstra et al., 2021). Here, we focused on the use of different APPs as markers for E. Coli infection. Table 1. Common APPs and their activities in the body.
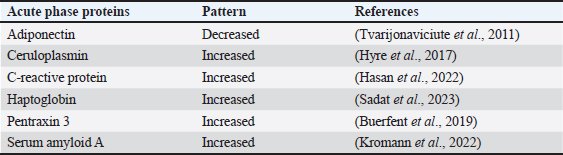
Table 2. List of common APP patterns associated with E. coli infection.
Acute phase proteins that are used as biomarkers for E. coli infectionAdiponectin Table 1 shows the activities of adiponectin as a member of AAPs. It has been shown that mice with knockout adiponectin showed a more severe inflammatory response and kidney damage than wild-type mice after E. coli infection; however, after administration of exogenous adiponectin, the inflammatory response was alleviated. Adiponectin is negatively correlated with CRP and haptoglobin (HP) during acute endotoxemia induced by E. coli (Tvarijonaviciute et al., 2011). Alterations in adiponectin levels are strongly correlated with the existence of normal flora in the intestines, which affects the normal health of the intestines in rats (Peng et al., 2020). Low levels of adiponectin are also correlated with higher E. coli content in the intestines of patients with prostate cancer and metabolic syndrome. Although adiponectin is related to E. coli infection and propagation, it could not be considered a specific marker for E. coli infection. Tables 3 and 4 demonstrate other involvements and associations in many microbial and non-infectious conditions.
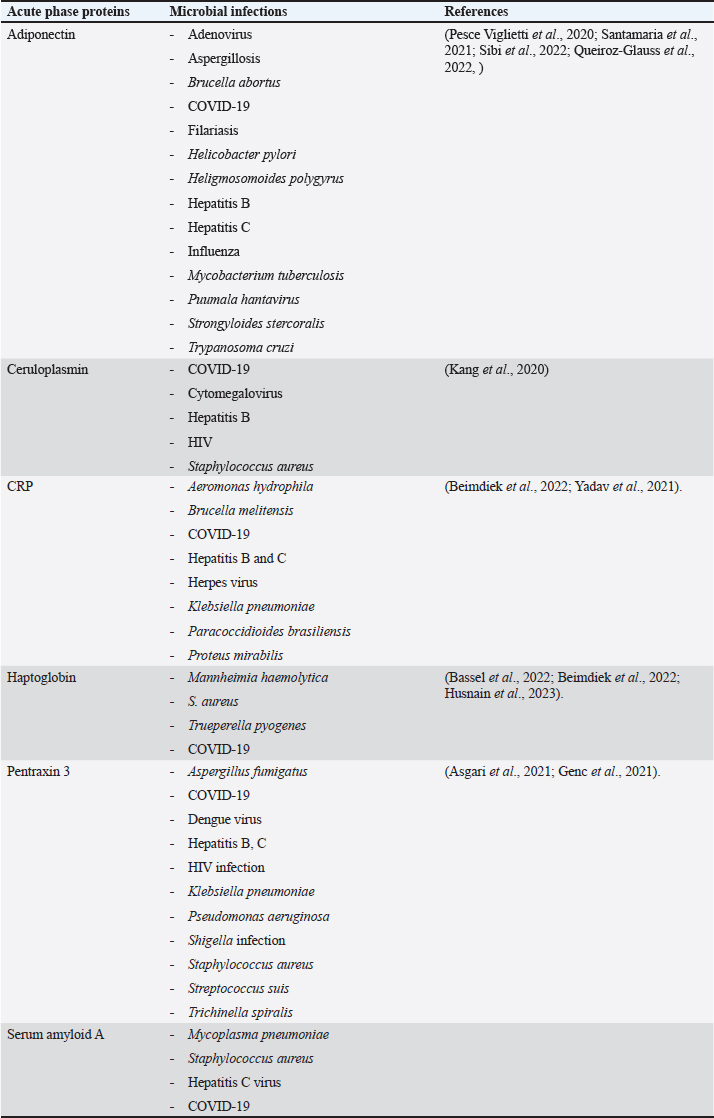
Fig. 1. Triangle shows that biomarkers can be specific for diagnosis and useful in monitoring, prediction, and prognosis of the disease. CeruloplasminCP is a copper transport protein that is involved in the regulation of copper and iron metabolism (Table 1). In addition, it has ferroxidase activity and is induced during inflammatory conditions due to infection (Liu et al., 2022). Recently, CP has been used as a biomarker to evaluate E. coli and Staphylococcus aureus infections (Sadat et al., 2023). CP levels have also been used to evaluate the bioactivity of silver nanoparticles against E. coli infection (Skomorokhova et al., 2020). CP was used to evaluate the effect of E. coli LPS challenge in animals. High levels of CP in the serum of E. coli (LPS)-inoculated animals were reduced by anti-inflammatory agents in a drug- and dose-dependent manner (Manzari Tavakoli et al., 2020). Although there was a relationship between the CP serum pattern and E. coli infection, it was not a unique marker for E. coli infection. Table 3 shows the involvement of CP in other bacterial and microbial infections. Accordingly, from the previously mentioned in Tables 3 and 4, CP is associated with E. coli and other microbial infections, as well as non-infectious inflammatory conditions. CP could not be considered a specific marker for E. coli infection but could be a marker for systemic inflammatory response. Table 3. APPs associated with microbial infections.
C-reactive proteinThe precise CRP activity levels are shown in Table 1. Clinically, CRP is widely used to assess inflammatory response, although some records have stated its unclear function (Cheng et al., 2022). Records have confirmed their role in the potentiation of phagocytosis (Kinoshita et al., 2021). Its serum level is widely used as a marker for infection/inflammation in emergency circumstances, point-of-care tests, and for the differentiation of viral and bacterial infections (Levinson and Wasserman, 2022). The serum level of CRP has been approved as a diagnostic marker for patients with sepsis and determines the level of E. Coli infection in patients with sepsis (Li et al., 2022). Mouse models have been used to elucidate the immune function of CRP in many trials to isolate and characterize endogenous CRP (Cheng et al., 2022). It has been used to differentiate between upper and lower UTIs induced by E. Coli; CRP levels were higher in upper than in lower UTIs, which enables determination of the anatomical position of UTIs and the method of therapy (Narayan Swamy et al., 2022). It was elevated in the sera of women with recurrent reproductive E. coli (Hasan et al., 2022). CRP is used as a biomarker for the prediction of bacteremia in children with febrile neutropenia, and it has been used with procalcitonin to predict bacterial infection in acute leukemic children (Nahar et al., 2023). It has also been used as a marker for the detection of neonatal sepsis induced by E. coli (Balayan et al., 2020). It can also be used to distinguish bloodstream infections from negative ones (Tang et al., 2020). In combination with WBC count, CRP level could be used as an improved diagnostic tool for gynecological and obstetric patients with infection (Jin et al., 2022). Although huge data have confirmed the association of high serum levels of CRP with E. coli infections, other data have also confirmed its association with other bacteria such as Streptococcus pyogenes (Germont et al., 2020), Proteus mirabilis, Staphylococcus lentus, and Citrobacter braakii (Lenicky et al., 2021). Regardless of the causative bacteria, CRP levels were increased in the sera of infected models, the matter which opened the debate for the ability to consider whether CRP is a specific marker for one species of bacteria or not. As previously mentioned, CRP can be considered a biomarker for the condition induced by the bacteria, not by the bacteria itself. Table 4. APPs associated with non-infectious conditions.
HaptoglobinAs shown in Table 1, HP is an APPs with antibacterial activity. Recent assays have been developed to detect only minute amounts of HP as a biomarker for E. coli infection (Nirala et al., 2020). A positive correlation between the number of E. coli colonies and HP serum levels was observed in animals infected with E. coli (Martin et al., 2021). HP has been approved for use in the differentiation between healthy and E. coli-infected animals; it started to be elevated in the sera of infected animals 4 days post-infection (Kromann et al., 2022). Similarly, E. coli enhances the serum levels of HP in infected animals (Wong et al., 2022, Husnain et al., 2023). In a transcriptomic study for the detection of differentially expressed genes between control and E. coli-infected animals, HP was upregulated in the infected groups. In contrast, administration of lipopolysaccharides derived from E. coli did not affect the level of HP in the serum of animals (Samarasinghe et al., 2020). Similarly, HP serum levels were not affected by fulminant necrotizing enterocolitis associated with E. coli infection in preterm infants (Nakamura et al., 2023). Moreover, mice affected by hemolytic uremic syndrome and deficient in HP showed low survival rates, and administration of a low dose of HP was associated with amelioration of kidney pathology (Pirschel et al., 2022). Serum HP was elevated 24 hours after administration of bacterial lysate formed from S. aureus and E. coli in the examined animals (Bassel et al., 2020). Recent clinical studies have revealed that HP was increased in the serum of animals suffering from mastitis induced by E. coli and S. aureus (Sadat et al., 2023). In contrast, HP levels declined in the serum of animals infected with the probiotic Bacillus subtilis strain as a result of the ameliorative effect of probiotics on the immune status of animals (He et al., 2020). The serum levels of HP were associated with other microbial infections and many inflammatory conditions, as shown in Tables 3 and 4. This matter dismisses its use as a specific marker for E. coli infections. Furthermore, the detection of HP phenotypes and using specific-phenotypic determinates are very important for linking HP levels to infection/inflammation conditions, and the concept of one fit for all is not recommended here (Skytthe et al., 2022). Pentraxin 3 (PTX3)Table 1 shows the activity of PTX3. Its antimicrobial power enables it to play a pivotal role in the defense against uropathogens (Miao and Abraham, 2014). Biochemically, it is a carbohydrate-binding protein of two domains, the N-terminal domain and the C-terminal domain, which is similar to CRP (Daigo and Hamakubo, 2020). The properties of PTX3 are similar to antibodies (Garlanda et al., 2016). Its high levels in the plasma of patients with bacteremia in the first days of infection encouraged its use as a potent prognostic marker (Huttunen et al., 2011). Regarding its use as a marker for E. coli infection, it has been detected that PTX3 secretion in the urine of humans and mice is markedly increased during UTIs (Burkhardt et al., 2019). In contrast, mice deficient in PTX3 lost their capacity to clear E. coli from their urinary tract. The authors reported that PTX3 secretion in urine was markedly enhanced in humans and mice following UTIs and that mice deficient in PTX3 were highly impaired in their capacity to clear uropathogenic E. coli following vesicular challenge (Jaillon et al., 2014). Moreover, high serum levels were associated with the prediction and diagnosis of COVID-19, hepatitis B, hepatitis C, and other microbial infections, as shown in Table 3. Accordingly, PTX3 could be considered a biomarker for predicting and diagnosing systemic inflammatory conditions (Table 4), regardless of the type of causative agent, whether E. coli or other microbial or non-microbial agents. Serum amyloid A (SAA)Table 1 shows the main activities of as APPs. It acts on many types of leukocytes in response to inflammation, infection, and/or injuries (Abouelasrar Salama et al., 2020). Clinically, it has been used as a potent marker of chronic inflammation (Zhang et al., 2019). Regarding its usage as a marker for E. coli infection, at the molecular level, its expression was increased in the conditions of E. coli infections (Murata et al., 2020) and its serum level could be used as a clinically sensitive biomarker for multiple inflammatory conditions induced by E. coli infections such as UTIs (Erman et al., 2012), septic arthritis at which its serum and the synovial level decreased with declining joint infection (Yoshimura et al., 2020), it has been approved as a potent marker for early diagnosis of neonatal septicemia (Balayan et al., 2020), endotoxemia (Esmaeili Seraji et al., 2022). Other studies have shown that the highest SAA serum level was between 4 and 6 days of infusion of E. coli (Esmaeili Seraji et al., 2022), and it has also been used as a marker in the diagnosis of E. coli bloodstream infection, pre-weaning diarrhea, and mastitis (Ahmed et al., 2021). On the other hand, SAA could be used as a marker for differentiation of the severity of inflammation in viral and bacterial infections, as its level is higher in viral than in bacterial infections (Aydin et al., 2022). Mammary gland infection with Escherichia coli-induced mastitis (Ahmed et al., 2021). Accordingly, SAA serum levels could be used clinically to predict and diagnose E. coli infections, although it is associated with other microbial and non-microbial inflammatory conditions (Tables 3 and 4). Studies that clinically associate SAA with infections are limited and need further consideration and approval (Su and Zhang, 2022). In general, SAA can be considered a marker for inflammatory conditions, regardless of etiology. ConclusionTo date, there are no unique specific biomarkers for E. coli infections. APPs can be used as markers of systemic inflammatory conditions originating from infection/inflammatory responses, regardless of the causative agent. Collectively, these patterns may aid in the early detection and prognosis of inflammatory responses. We do not recommend APPs as diagnostic, prognostic, or predictive markers for E. coli infection, so further studies are recommended to identify a marker for E. coli or other bacterial infections. AcknowledgmentsThe author is thankful to the Deanship of Graduate Studies and Scientific Research at University of Bisha for supporting this work through the Fast-Track Research Support Program. Conflict of interestThe author declares no conflict of interest. FundingNo funding. Authors’ contributionsThe authors have equal participation in conceptualization, writing the original draft preparation, writing reviews, and editing. Data availabilityAll data analyzed are included in this review article. ReferencesAbouelasrar Salama, S., De Bondt, M., De Buck, M., Berghmans, N., Proost, P., Oliveira, V. L.S., Amaral, F.A., Gouwy, M., Van Damme, J. and Struyf, S. 2020. Serum amyloid A1 (Saa1) revisited: restricted leukocyte-activating properties of homogeneous Saa1. Front. Immunol. 11, 843. Ahmed, H.F., Hegazy, Y.M. and Ibrahem, S.A. 2021. Interrelationship of milk acute-phase proteins and casein percentage in cows and buffaloes subclinical mastitis. Vet. Res. Forum. 12, 409–414. Asgari, F., Supino, D., Parente, R., Polentarutti, N., Stravalaci, M., Porte, R., Pasqualini, F., Barbagallo, M., Perucchini, C., Recordati, C., Magrini, E., Mariancini, A., Riva, F., Giordano, A., Davoudian, S., Roger, T., Veer, C.V., Jaillon, S., Mantovani, A., Doni, A. and Garlanda, C. 2021. The long pentraxin Ptx3 controls klebsiella pneumoniae severe infection. Front. Immunol. 12, 666198. Aydin, O., Ulas, N., Genc, A., Baysal, S., Kandemir, O. and Aktas, M.S. 2022. Investigation of hemogram, oxidative stress, and some inflammatory marker levels in neonatal calves with Escherichia coli and coronavirus diarrhea. Microb. Pathog. 173, 105802. Balayan, S., Chauhan, N., Chandra, R., Kuchhal, N.K. and Jain, U. 2020. Recent advances in developing biosensing based platforms for neonatal sepsis. Biosens. Bioelectron. 169, 112552. Balikci, E. and Al, M. 2014. Some serum acute phase proteins and immunoglobulins concentrations in calves with rotavirus, coronavirus, E. coli F5 and Eimeria species. Iran. J. Vet. Res. 15, 397–401. Barichello, T., Rocha Catalao, C.H., Rohlwink, U.K., Van Der Kuip, M., Zaharie, D., Solomons, R.S., Van Toorn, R., Tutu Van Furth, M., Hasbun, R., Iovino, F. and Namale, V.S. 2023. Bacterial meningitis in Africa. Front. Neurol. 14, 822575. Bassel, L.L., Co, C., Macdonald, A., Sly, L., Mccandless, E.E., Hewson, J., Tiwari, R., Sharif, S., Siracusa, L., Clark, M.E. and Caswell, J.L. 2020. Pulmonary and systemic responses to aerosolized lysate of Staphylococcus aureus and Escherichia coli in calves. BMC Vet. Res. 16, 168. Bassel, L.L., Kaufman, E.I., Alsop, S.N.A., Sergejewich, L., Vulikh, K., Stinson, K.J., Siracusa, L.R., Buchan, J., Hewson, J., Sharif, S. and Caswell, J.L. 2022. The effect of aerosolized bacterial lysate on experimentally induced mannheimia haemolytica pneumonia in calves. Can. J. Vet. Res. 86, 85–92. Beimdiek, J., Janciauskiene, S., Wrenger, S., Volland, S., Rozy, A., Fuge, J., Olejnicka, B., Pink, I., Illig, T., Popov, A., Chorostowska, J., Buettner, F.F.R. and Welte, T. 2022. Plasma markers of Covid-19 severity: a pilot study. Respir. Res. 23, 343. Buerfent, B.C., Golz, L., Hofmann, A., Ruhl, H., Stamminger, W., Fricker, N., Hess, T., Oldenburg, J., Nothen, M.M., Schumacher, J., Hubner, M.P. and Hoerauf, A. 2019. Transcriptome-wide analysis of filarial extract-primed human monocytes reveal changes in LPS-induced PTX3 expression levels. Sci. Rep. 9, 2562. Burkhardt, N.B., Roll, S., Staudt, A., Elleder, D., Hartle, S., Costa, T., Alber, A., Stevens, M. P., Vervelde, L., Schusser, B. and Kaspers, B. 2019. The long pentraxin PTX3 is of major importance among acute phase proteins in chickens. Front. Immunol. 10, 124. Chae, W.R., Nubel, J., Baumert, J., Gold, S.M. and Otte, C. 2022. Association of depression and obesity with C-reactive protein in Germany: a large nationally representative study. Brain Behav. Immun. 103, 223–231. Cheng, B., Wu, D., Wu, K., Huang, X.P., Lv, J.M., Ji, S.R. and Zhu, L. 2022. Purification of recombinant mouse C-reactive protein from pichia pastoris Gs115 by nickel chelating sepharose fast-flow affinity chromatography and p-aminophenyl phosphoryl choline agarose resin affinity chromatography in tandem. J. Chromatogr. Sci. 60, 750–759. Cobo-Simon, M., Hart, R. and Ochman, H. 2023. Escherichia coli: what is and which are? Mol. Biol. Evol. 40(1), msac273. Coe, S.E., Magagna, M.A., Zimmerman, A., George, A., Carter, C., Dean, C. and Nelson, K. 2022. Extraintestinal pathogenic Escherichia coli causes necrohemorrhagic pneumonia in multiple research dogs. Toxicol. Pathol. 50, 904–909. Daigo, K. and Hamakubo, T. 2020. Expression and purification of full-length and domain-fragment recombinant pentraxin 3 (PTX3) proteins from mammalian and bacterial cells. Methods Mol. Biol. 2132, 65–74. Erman, A., Lakota, K., Mrak-Poljsak, K., Blango, M.G., Krizan-Hergouth, V., Mulvey, M.A., Sodin-Semrl, S. and Veranic, P. 2012. Uropathogenic Escherichia coli induces serum amyloid a in mice following urinary tract and systemic inoculation. PLoS One 7, E32933. Esmaeili Seraji, R., Chalmeh, A. and Pourjafar, M. 2022. Low molecular weight heparin reduces acute phase response and multiple organ dysfunction following ovine experimental endotoxemia model. Vet. Immunol. Immunopathol. 243, 110361. Garlanda, C., Jaillon, S., Doni, A., Bottazzi, B. and Mantovani, A. 2016. PTX3, a humoral pattern recognition molecule at the interface between microbe and matrix recognition. Curr. Opin. Immunol. 38, 39-44. Genc, A.B., Yaylaci, S., Dheir, H., Genc, A.C., Issever, K., Cekic, D., Kocayigit, H., Cokluk, E., Karacan, A., Sekeroglu, M.R., Toptan Cakar, H. and Guclu, E. 2021. The predictive and diagnostic accuracy of long pentraxin-3 in Covid-19 pneumonia. Turk. J. Med. Sci. 51, 448–453. Germont, Z., Bidet, P., Plainvert, C., Bonacorsi, S., Poyart, C., Biran, V., Frerot, A., Faye, A. and Basmaci, R. 2020. Invasive streptococcus pyogenes infections in <3-month-old infants in France: clinical and laboratory features. Front. Pediatr. 8, 204. Goncales, R.A., Bastos, H.N., Duarte-Oliveira, C., Antunes, D., Sokhatska, O., Jacob, M., Rolo, R., Campos, C.F., Sasaki, S.D., Donato, A., Mapelli, S.N., Costa, S., Moura, C.S., Delgado, L., Morais, A., Torrado, E., Van De Veerdonk, F.L., Weichhart, T., Lambris, J. D., Silvestre, R., Garlanda, C., Mantovani, A., Cunha, C. and Carvalho, A. 2022. Pentraxin 3 inhibits complement-driven macrophage activation to restrain granuloma formation in sarcoidosis. Am. J. Respir. Crit. Care Med. 206, 1140–1152. Hasan, S.F., Al-Hashemi, I.H.M., Al-Rammahi, T.M.M. and Abbas, Z.F. 2022. Interleukin-6 and C-reactive protein: their association with vitamin D in women with recurrent infections of reproductive system. Egypt. J. Immunol. 29, 1–8. Hashimoto, R., Shoji, K., Ishiguro, A. and Miyairi, I. 2022. Clinical characteristics of bacteremic urinary tract infection due to third-generation cephalosporin-resistant Escherichia coli in children. J. Infect. Chemother. 28, 469–471. He, Y., Kim, K., Kovanda, L., Jinno, C., Song, M., Chase, J., Li, X., Tan, B. and Liu, Y. 2020. Bacillus subtilis: a potential growth promoter in weaned pigs in comparison to carbadox. J. Anim. Sci. 98(9), skaa290. He, Z., Liu, J.J., Shu, G.F., Ma, S.L. and Wu, G.Q. 2022. Diagnostic utility of Cd64 and Cd38 biomarkers for the differential diagnosis of infections. Genet. Test. Mol. Biomarkers 26, 133–139. Husnain, A., Arshad, U., Poindexter, M.B., Zimpel, R., Marinho, M.N., Perdomo, M.C., Fan, P., Jeong, K.C., Nelson, C.D., Sheldon, I.M., Bromfield, J.J. and Santos, J.E.P. 2023. Induced endometritis in early lactation compromises production and reproduction in dairy cows. J. Dairy Sci. 106(6), 4198–4213. Huttunen, R., Hurme, M., Aittoniemi, J., Huhtala, H., Vuento, R., Laine, J., Jylhava, J. and Syrjanen, J. 2011. High plasma level of long pentraxin 3 (PTX3) is associated with fatal disease in bacteremic patients: a prospective cohort study. PLoS One 6, e17653. Hyre, A.N., Kavanagh, K., Kock, N.D., Donati, G.L. and Subashchandrabose, S. 2017. Copper is a host effector mobilized to urine during urinary tract infection to impair bacterial colonization. Infect. Immun. 85(3), e01041-16. Jaffey, J.A., Su, D., Monasky, R., Hanratty, B., Flannery, E. and Horman, M. 2022. Effects of a whole food diet on immune function and inflammatory phenotype in healthy dogs: a randomized, open-labeled, cross-over clinical trial. Front. Vet. Sci. 9, 898056. Jaillon, S., Moalli, F., Ragnarsdottir, B., Bonavita, E., Puthia, M., Riva, F., Barbati, E., Nebuloni, M., Cvetko Krajinovic, L., Markotic, A., Valentino, S., Doni, A., Tartari, S., Graziani, G., Montanelli, A., Delneste, Y., Svanborg, C., Garlanda, C. and Mantovani, A. 2014. The humoral pattern recognition molecule PTX3 is a key component of innate immunity against urinary tract infection. Immunity 40, 621–632. Jin, J., Fan, X., Dong, X., Zhai, X., Ma, Y. and Tang, J. 2022. Infection and the evaluation of biomarkers in obstetrics and gynecology patients with infectious disease: a retrospective observational study from clinical pharmacists’ consultation Experience. Eur. J. Med. Res. 27, 229. Jonasdottir, A.D., Antovic, A., Qureshi, A.R., Nordin, A., Malmstrom, V., Gunnarsson, I. and Bruchfeld, A. 2023. Pentraxin-3—a potential biomarker in anca-associated vasculitis. scand. J. Rheumatol. 52, 293–301. Kang, N.L., Zhang, J.M., Liu, Y.R., Lin, S., Dong, J., Jiang, J.J., Zhu, Y.Y. and Zeng, D.W. 2020. Novel predictive models using serum ceruloplasmin levels for hepatic steatosis in patients with Chronic hepatitis B infection. Clin. Res. Hepatol. Gastroenterol. 44, 57–65. Karam, S., Haidous, M., Royal, V. and Leung, N. 2023. Renal AA amyloidosis: presentation, diagnosis, and current therapeutic options: a review. Kidney Int. 103, 473–484. Karim, A., Muhammad, T., Shah, I., Khan, J. and Qaisar, R. 2022. Relationship of haptoglobin phenotypes with sarcopaenia in patients with congestive heart failure. Heart Lung Circ. 31, 822–831. Khudiakova, A.D., Polonskaya, Y.V., Shramko, V.S., Shcherbakova, L.V., Striukova, E.V., Kashtanova, E.V. and Ragino, Y.I. 2022. Blood adipokines/cytokines in young people with chronic bronchitis and abdominal obesity. Biomolecules 12(10), 1502. Kinoshita, M., Ito, S., Ishikiriyama, T., Sekiguchi, K., Yamaguchi, R., Tsuruhara, R., Matsuda, A., Koiwa, K., Nakashima, M., Nakashima, H., Miyashita, M. and Seki, S. 2021. The efficacy of posttreatment with synthetic C-reactive protein in murine bacterial peritonitis via activation of fcgammari-expressing kupffer cells. J. Innate Immun. 13, 306–318. Kromann, S., Olsen, R.H., Bojesen, A.M., Jensen, H.E. and Thofner, I. 2022. Assessment of automated assays for serum amyloid A, haptoglobin (PIT54) and basic biochemistry in broiler breeders experimentally infected with Escherichia coli. Vet. Res. 53, 25. Ku, L.C., Boggess, K.A. and Cohen-Wolkowiez, M. 2015. Bacterial meningitis in infants. Clin. Perinatol. 42, 29–45, Vii–Viii. Kunc, P., Fabry, J., Lucanska, M. and Pecova, R. 2020. Biomarkers of bronchial asthma. Physiol. Res. 69, S29–S34. Kushner, I. 2023. C-reactive protein—My perspective on its first half century, 1930–1982. Front. Immunol. 14, 1150103. Leimbach, A., Hacker, J. and Dobrindt, U. 2013. E. coli as an all-rounder: the thin line between commensalism and pathogenicity. Curr. Top. Microbiol. Immunol. 358, 3–32. Lenicky, M., Slanina, T., Kacaniova, M., Galovicova, L., Petrovicova, M., Duracka, M., Benko, F., Kovac, J. and Tvrda, E. 2021. Identification of bacterial profiles and their interactions with selected quality, oxidative, and immunological parameters of Turkey semen. Animals (Basel) 11(6), 1771. Levinson, T. and Wasserman, A. 2022. C-reactive protein velocity (CRPv) as a new biomarker for the early detection of acute infection/inflammation. Int. J. Mol. Sci. 23(15), 8100. Li, J., Hu, L. and Li, L. 2022. C-reactive protein, procalcitonin, and a novel pathogenesis and therapeutic target of thrombocytopenia in sepsis. Emerg. Med. Int. 2022, 2498435. Liu, Z., Wang, M., Zhang, C., Zhou, S. and Ji, G. 2022. Molecular functions of ceruloplasmin in metabolic disease pathology. Diabetes Metab. Syndr. Obes. 15, 695–711. Mackellar, M. and Vigerust, D.J. 2016. Role of haptoglobin in health and disease: a focus on diabetes. Clin. Diabetes 34, 148–57. Manzari Tavakoli, M., Abdi-Hachesoo, B., Nazifi, S., Mosleh, N., Hosseinian, S.A. and Nakhaee, P. 2020. Comparative effects of dexamethasone and meloxicam on magnitude of the acute inflammatory response induced by Escherichia coli lipopolysaccharide in broiler chickens. J. Inflamm. Res. 13, 487–495. Martin, C.C., Baccili, C.C., Avila-Campos, M.J., Hurley, D.J. and Gomes, V. 2021. Effect of prophylactic use of tulathromycin on gut bacterial populations, inflammatory profile and diarrhea in newborn holstein calves. Res. Vet. Sci. 136, 268–276. Meacham, C.E., Jeffery, E.C., Burgess, R.J., Sivakumar, C.D., Arora, M.A., Stanley, A.M., Colby, E.M., Crane, G.M., Zhao, Z. and Morrison, S.J. 2022. Adiponectin receptors sustain haematopoietic stem cells throughout adulthood by protecting them from inflammation. Nat. Cell Biol. 24, 697–707. Miao, Y. and Abraham, S.N. 2014. Peeing pentraxins. Immunity 40, 460–462. Murata, E., Kozaki, S., Murakami, T., Shimizu, K., Okada, A., Ishiguro, N. and Inoshima, Y. 2020. Differential expression of serum amyloid A1 and A3 in bovine epithelia. J. Vet. Med. Sci. 82, 764–770. Muzembo, B.A., Kitahara, K., Ohno, A., Okamoto, K. and Miyoshi, S.I. 2022. Colonization with extended-spectrum beta-lactamase-producing Escherichia coli and traveler’s diarrhea attack rates among travelers to India: a systematic review and meta-analysis. Trop. Dis. Travel Med. Vaccines 8, 22. Nahar, A., Jamal, C.Y., Refat, R., Chowdhury, T., Akter, S., Karim, A., Rahman, M.A., Yeamin, M.B., Saha, B.K., Hossain, F. and Rabbany, M.A. 2023. Procalcitonin versus C-reactive protein as a biomarker for prediction of bacterial infection in children with febrile neutropenia in acute leukemia. Mymensingh. Med. J. 32, 76–82. Nakamura, T., Inoue, S., Ito, K., Fukama, E., Nomura, T., Hatanaka, D., Kusakari, M., Takahashi, H. and Yamada, S. 2023. Investigation of biomarkers in a rare case of fulminant necrotizing enterocolitis in a preterm infant. Fukushima J. Med. Sci. 69, 29–36. Narayan Swamy, S.N., Jakanur, R.K. and Sangeetha, S.R. 2022. Significance of C-reactive protein levels in categorizing upper and lower urinary tract infection in adult patients. Cureus 14, e26432. Nirala, N.R., Harel, Y., Lellouche, J.P. and Shtenberg, G. 2020. Ultrasensitive haptoglobin biomarker detection based on amplified chemiluminescence of magnetite nanoparticles. J. Nanobiotechnology 18, 6. Nyirenda, M.H., Fadda, G., Healy, L.M., Mexhitaj, I., Poliquin-Lasnier, L., Hanwell, H., Saveriano, A.W., Rozenberg, A., Li, R., Moore, C.S., Belabani, C., Johnson, T., O’mahony, J., Arnold, D.L., Yeh, E.A., Marrie, R.A., Dunn, S., Banwell, B. and Bar-Or, A. 2021. Pro-inflammatory adiponectin in pediatric-onset multiple sclerosis. Mult. Scler. 27, 1948–1959. Peng, Y., Zhang, N., Li, W.J., Tan, K., Zhou, Y., She, C. and Chen, H.N. 2020. Correlations of changes in inflammatory factors, glucose and lipid metabolism indicators and adiponectin with alterations in intestinal flora in rats with coronary heart disease. Eur. Rev. Med. Pharmacol. Sci. 24, 10118–10125. Pesce Viglietti, A.I., Giambartolomei, G.H., Quarleri, J. and Delpino, M.V. 2020. Brucella abortus infection modulates 3T3-L1 adipocyte inflammatory response and inhibits adipogenesis. Front. Endocrinol. (Lausanne) 11, 585923. Pirschel, W., Mestekemper, A.N., Wissuwa, B., Krieg, N., Kroller, S., Daniel, C., Gunzer, F., Tolosano, E., Bauer, M., Amann, K., Heinemann, S.H. and Coldewey, S.M. 2022. Divergent roles of haptoglobin and hemopexin deficiency for disease progression of shiga-toxin-induced hemolytic-uremic syndrome in mice. Kidney Int. 101, 1171–1185. Queiroz-Glauss, C.P., Vieira, M.S., Goncalves-Pereira, M.H., Almeida, S.S., Freire, R.H., Gomes, M.A., Alvarez-Leite, J.I. and Santiago, H.C. 2022. Helminth infection modulates number and function of adipose tissue tregs in high fat diet-induced obesity. PLoS Negl. Trop. Dis. 16, e0010105. Raia, S., Conti, A., Zanardi, A., Ferrini, B., Scotti, G.M., Gilberti, E., De Palma, G., David, S. and Alessio, M. 2023. Ceruloplasmin-deficient mice show dysregulation of lipid metabolism in liver and adipose tissue reduced by a protein replacement. Int. J. Mol. Sci. 24(2), 1150. Rumball, N.A., Alm, E.W. and Mclellan, S.L. 2023. Genetic determinants of Escherichia coli survival in beach sand. Appl. Environ. Microbiol. 89, e0142322. Sack, G.H., Jr. 2020. Serum amyloid A (SAA) proteins. Subcell. Biochem. 94, 421–436. Sadat, A., Farag, A.M.M., Elhanafi, D., Awad, A., Elmahallawy, E.K., Alsowayeh, N., El-Khadragy, M.F. and Elshopakey, G.E. 2023. Immunological and oxidative biomarkers in bovine serum from healthy, clinical, and sub-clinical mastitis caused by Escherichia coli and Staphylococcus aureus infection. Animals (Basel) 13(5), 892. Samarasinghe, M.B., Sehested, J., Larsen, T. and Hernandez-Castellano, L.E. 2020. Oral administration of lipopolysaccharides from Escherichia coli (Serotype O111:B4) does not induce an effective systemic immune response in milk-fed holstein calves. J. Dairy Sci. 103, 5525–5531. Sammel, A.M., Xue, M., Karsten, E., Little, C.B., Smith, S., Nguyen, K. and Laurent, R. 2021. Limited utility of novel serological biomarkers in patients newly suspected of having giant cell arteritis. Int. J. Rheum. Dis. 24, 781–788. Santamaria, M.H., Rios, L.D. and Corral, R.S. 2021. Trypanosoma cruzi down-regulates adiponectin expression in mouse adipocytes via the NFAT signaling pathway. Microbes. Infect. 23, 104757. Sheikh, A. and Fleckenstein, J.M. 2023. Interactions of pathogenic Escherichia coli with ceacams. Front. Immunol. 14, 1120331. Shi, W., Wu, Y., Zhong, L., Huang, Z., Zhong, M., Wang, J., Huang, W., Yi, X., Lu, X., Yuan, J., Huang, W. and Zhou, X. 2022. Diagnostic accuracy of serum amyloid a in acute appendicitis: a systematic review and meta-analysis. Surg. Infect. (Larchmt) 23, 380–387. Shklyaev, S.S., Melnichenko, G.A., Volevodz, N.N., Falaleeva, N.A., Ivanov, S.A., Kaprin, A.D. and Mokrysheva, N.G. 2021. Adiponectin: a pleiotropic hormone with multifaceted roles. Probl. Endokrinol. (Mosk) 67, 98–112. Sibi, J.M., Mohan, V., Deepa, M., Babu, S. and Aravindhan, V. 2022. Modulatory effect of filarial infection on the systemic hormone levels in subjects with metabolic syndrome (DM-LF5). Front. Endocrinol. (Lausanne) 13, 1011942. Skomorokhova, E.A., Sankova, T.P., Orlov, I.A., Savelev, A.N., Magazenkova, D.N., Pliss, M.G., Skvortsov, A.N., Sosnin, I.M., Kirilenko, D.A., Grishchuk, I.V., Sakhenberg, E.I., Polishchuk, E.V., Brunkov, P.N., Romanov, A.E., Puchkova, L.V. and Ilyechova, E.Y. 2020. Size-dependent bioactivity of silver nanoparticles: antibacterial properties, influence on copper status in mice, and whole-body turnover. Nanotechnol. Sci. Appl. 13, 137–157. Skytthe, M.K., Sorensen, A.L., Hennig, D., Sandberg, M.B., Rasmussen, L.M., Moller, H.J., Skjodt, K., Graversen, J.H. and Moestrup, S.K. 2022. Re-evalution of the measurement of haptoglobin in human plasma samples. Scand. J. Clin. Lab. Invest. 82, 467–473. Soric Hosman, I., Kos, I. and Lamot, L. 2020. Serum amyloid a in inflammatory rheumatic diseases: a compendious review of a renowned biomarker. Front. Immunol. 11, 631299. Su, M. and Zhang, L. 2022. Research status of serum amyloid a in infection: a bibliometric analysis. Ann. Palliat. Med. 11, 2007–2016. Suzuki, S., Katagiri, R., Yamaji, T., Sawada, N., Imatoh, T., Ihira, H., Inoue, M., Tsugane, S., Iwasaki, M. and Japan Public Health Center-Based Prospective Study Group. 2022. Association between C-reactive protein and risk of overall and 18 site-specific cancers in a Japanese case-cohort. Br. J. Cancer 126, 1481–1489. Tang, W., Zhang, W., Li, X., Cheng, J., Liu, Z., Zhou, Q. and Guan, S. 2020. Hematological parameters in patients with bloodstream infection: a retrospective observational study. J. Infect. Dev. Ctries. 14, 1264–1273. Thomas-Dupont, P., Velazquez-Soto, H., Izaguirre-Hernandez, I.Y., Amieva-Balmori, M., Triana-Romero, A., Islas-Vazquez, L., Jimenez-Martinez, M.D.C. and Remes-Troche, J. M. 2022. Obesity contributes to inflammation in patients with ibs via complement component 3 and C-reactive protein. Nutrients 14(24), 5227. Tong, S., Jiang, N., Wan, J.H., Chen, C.R., Wang, S.H., Wu, C.Y., Guo, Q., Xiao, X.Y., Huang, H. and Zhou, T. 2023. The effects of the prognostic biomarker SAAL1 on cancer growth and its association with the immune microenvironment in lung adenocarcinoma. BMC Cancer 23, 275. Tvarijonaviciute, A., Eralp, O., Kocaturk, M., Yilmaz, Z. and Ceron, J.J. 2011. Adiponectin and IGF-1 are negative acute phase proteins in a dog model of acute endotoxaemia. Vet. Immunol. Immunopathol. 140, 147–151. Voros, K., Markus, B., Atzel, K., Szalay, F., Graf, L., Nemeth, D., Masszi, T., Torzsa, P. and Kalabay, L. 2023. Serum fetuin-A is decreased in cirrhotic patients with Wilson’s disease. PLoS One 18, e0282801. Vural, S. and Albayrak, L. 2022. Can calcitonin gene-related peptide (CGRP) and pentraxin-3 (PTX-3) be useful in diagnosing acute migraine attack? J. Recept. Signal. Transduct. Res. 42, 562–566. Wong, B.T., Park, S., Kovanda, L., He, Y., Kim, K., Xu, S., Lingga, C., Hejna, M., Wall, E., Sripathy, R., Li, X. and Liu, Y. 2022. Dietary supplementation of botanical blends enhanced performance and disease resistance of weaned pigs experimentally infected with enterotoxigenic Escherichia coli F18. J. Anim. Sci. 100(12), skac353. Yadav, S.K., Dash, P., Sahoo, P.K., Garg, L.C. and Dixit, A. 2021. Recombinant outer membrane protein ompc induces protective immunity against aeromonashydrophila infection in labeorohita. Microb. Pathog. 150, 104727. Yates, J. 2005. Traveler’s diarrhea. Am. Fam. Physician. 71, 2095–2100. Yin, J., Zeng, H., Fan, K., Xie, H., Shao, Y., Lu, Y., Zhu, J., Yao, Z., Liu, L., Zhang, H., Luo, B., Wang, X., Zeng, C., Bai, X., Zhang, H. and Cai, D. 2022. Pentraxin 3 regulated By miR-224-5p modulates macrophage reprogramming and exacerbates osteoarthritis associated synovitis by targeting CD32. Cell Death Dis. 13, 567. Yoshimura, S., Koziy, R.V., Dickinson, R., Moshynskyy, I., Mckenzie, J.A., Simko, E. and Bracamonte, J.L. 2020. Use of serum amyloid a in serum and synovial fluid to detect eradication of infection in experimental septic arthritis in horses. Can. J. Vet. Res. 84, 198–204. Zandstra, J., Jongerius, I. and Kuijpers, T.W. 2021. Future biomarkers for infection and inflammation in febrile children. Front. Immunol. 12, 631308. Zhang, P., Jiang, N., Xu, L., Shen, Z., Liu, X. and Cai, X. 2022. Clostridium perfringens and Escherichia coli bacteremia in a patient with acute obstructive suppurative cholangitis: a case report and review of the literature. Am. J. Case Rep. 23, e936329. Zhang, Y., Zhang, J., Sheng, H., Li, H. and Wang, R. 2019. Acute phase reactant serum amyloid A in inflammation and other diseases. Adv. Clin. Chem. 90, 25–80. Zhou, A. and Hypponen, E. 2023. Vitamin D deficiency and C-reactive protein: a bidirectional mendelian randomization study. Int. J. Epidemiol. 52, 260–271. | ||
| How to Cite this Article |
| Pubmed Style Ali AA, Darwish WS. Acute phase proteins patterns as biomarkers in bacterial infection: Recent insights. Open Vet. J.. 2024; 14(10): 2539-2550. doi:10.5455/OVJ.2024.v14.i10.4 Web Style Ali AA, Darwish WS. Acute phase proteins patterns as biomarkers in bacterial infection: Recent insights. https://www.openveterinaryjournal.com/?mno=214121 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i10.4 AMA (American Medical Association) Style Ali AA, Darwish WS. Acute phase proteins patterns as biomarkers in bacterial infection: Recent insights. Open Vet. J.. 2024; 14(10): 2539-2550. doi:10.5455/OVJ.2024.v14.i10.4 Vancouver/ICMJE Style Ali AA, Darwish WS. Acute phase proteins patterns as biomarkers in bacterial infection: Recent insights. Open Vet. J.. (2024), [cited January 25, 2026]; 14(10): 2539-2550. doi:10.5455/OVJ.2024.v14.i10.4 Harvard Style Ali, A. A. & Darwish, . W. S. (2024) Acute phase proteins patterns as biomarkers in bacterial infection: Recent insights. Open Vet. J., 14 (10), 2539-2550. doi:10.5455/OVJ.2024.v14.i10.4 Turabian Style Ali, Amer Al, and Wageh Sobhy Darwish. 2024. Acute phase proteins patterns as biomarkers in bacterial infection: Recent insights. Open Veterinary Journal, 14 (10), 2539-2550. doi:10.5455/OVJ.2024.v14.i10.4 Chicago Style Ali, Amer Al, and Wageh Sobhy Darwish. "Acute phase proteins patterns as biomarkers in bacterial infection: Recent insights." Open Veterinary Journal 14 (2024), 2539-2550. doi:10.5455/OVJ.2024.v14.i10.4 MLA (The Modern Language Association) Style Ali, Amer Al, and Wageh Sobhy Darwish. "Acute phase proteins patterns as biomarkers in bacterial infection: Recent insights." Open Veterinary Journal 14.10 (2024), 2539-2550. Print. doi:10.5455/OVJ.2024.v14.i10.4 APA (American Psychological Association) Style Ali, A. A. & Darwish, . W. S. (2024) Acute phase proteins patterns as biomarkers in bacterial infection: Recent insights. Open Veterinary Journal, 14 (10), 2539-2550. doi:10.5455/OVJ.2024.v14.i10.4 |