| Original Article | ||
Open Vet. J.. 2022; 12(4): 481-488 Open Veterinary Journal, (2022), Vol. 12(4): 481–488 Original Research Gastrointestinal parasites of baboons (Papio papio) in Niokolo-Koba National Park, SenegalKacou Martial N’da1*, Laibané Dieudonné Dahourou2,3, Papa Ibnou Ndiaye4, Stacy Lindshield5, Oubri Bassa Gbati1 and Amadou Traore31Département Santé Publique—Environnement, Ecole Inter-Etats des Sciences et Médecine Vétérinaires de Dakar, Dakar, Sénégal 2Département de l’élevage, Institut des Sciences de l’Environnement et du Développement rural, Université de Dédougou, Dédougou, Burkina Faso 3Laboratoire de Biologie et Santés Animales (LABIOSA), Institut de l’Environnement et de Recherches Agricoles (INERA), Ouagadougou, Burkina Faso 4Département de Biologie Animale, Faculté des Sciences et Techniques, Université Cheikh Anta Diop de Dakar, Senegal 5Anthropology Department, Purdue University, West Lafayette, IN, USA *Corresponding Author: Kacou Martial N’da. Département Santé Publique—Environnement, Ecole Inter-Etats des Sciences et Médecine Vétérinaires de Dakar, Dakar, Sénégal. Email: ndakacoumartial [at] gmail.com Submitted: 08/02/2022 Accepted: 24/06/2022 Published: 15/07/2022 © 2022 Open Veterinary Journal
AbstractBackground: Primates can harbor parasites that could be pathogenic or not for humans and primates themselves. It is necessary to know the parasitological situation of the primates that are under surveillance in the park. Aim: To estimate the prevalence and diversity of gastrointestinal parasites, including zoonotic potential parasites, in baboons in the Niokolo-Koba National Park located in Senegal. Method: Fecal samples (n=50) from two groups of baboons (A and B) were collected in October 2019. The samples were processed using the flotation technique and the modified Ritchie method. Slides were examined microscopically and the parasite identification was based on morphology, color, and parasite content. Results: A total of seven nematodes (Strongyloides sp., Trichirus sp., Ancylostoma sp., Mammo monogamus, Enterobius sp., Strongyloides stercoralis, Strongyle digestif), one cestode (Bertiella sp.), and one trematode (Fasciolopsis sp.) were identified. The overall prevalence was 78%, while the prevalence of poly-infected samples was 49%. The parasite with zoonotic potential, S. stercoralis, was identified in group B samples. Trichuris sp., which is common and pathogenic to humans and primates, was present with prevalence of 52% and of 32% in groups A and B, respectively. Conclusion: These results suggest that baboons are infested with zoonotic parasites and this situation could expose people working in this park to infection. Contact between humans and these baboons or their feces could expose them to infection with zoonotic parasites. Keywords: Niokolo-Koba National Park, Baboons, Gastrointestinal parasites, Zoonoses. IntroductionGastrointestinal parasitism in baboons is fairly well documented in desert areas (Murray et al., 2000; Hahn et al., 2003; Ocaido et al., 2003; Legesse and Erko, 2004). However, research on gastrointestinal parasites of baboons in forest areas is quite limited. An understanding of the variability in parasite infection patterns of baboons in the desert, savanna, and forest habitats could improve knowledge of the role of climatic variation and habitat structure in determining the infection patterns observed in a given geographic area. Climate, concurrent infections, and seasonality are factors described in previous studies as affecting the prevalence, intensity, and severity of parasitic infestations in animals (Messager, 2018). Some intestinal parasites are among the most important parasites of humans, as well as other nonhuman primates, particularly the major soil-transmitted helminthes, such as Ascaris lumbricoides, Trichuris trichiura, Strongyloides stercoralis, and hookworms that are members of the strongyles group. A study of baboons in the Bugwe Forest Reserve in Uganda showed a fairly high prevalence of Strongyloides sp. (60.7%) and Trichostrongylus sp. (Ocaido et al., 2003). However, variations in prevalence were noted from one geographical region to another. McGrew et al. (1989) compared the parasites of the Babouis at the level of Mount Assirik in Senegal and those of Gombe park in Tanzania. The findings indicated that the baboons of Gombe were more often infected than those of Mount Assirik. On the contrary, recent studies conducted on primates, by N'da et al. (2021) in the Bandia Reserve and by Howells et al. (2011) in Fongoli, have shown that monkeys and baboons harbor many zoonotic parasites, indicating a risk of transmission to humans. Indeed, several zoonotic parasites, including Entamoeba histolytica are responsible for more than 55,000 deaths per year (Ghosh et al., 2019). Giardia duodenalis is a parasite found in primates and humans that can cause serious health problems in humans (Dixon, 2021). Other protozoa, such as Blastocystis hominis and Dientamoeba fragilis, also have pathogenicity and can be transmitted from primates to humans. Hymenolepis nana, Ancylostoma duodenale, and S. stercoralis are nematodes, recognized parasites with zoonotic potential, which can cause serious digestive disorders in humans, if infection with these parasites is not treated quickly (WHO, 1987). Indeed, the similarities in physiology, behavior, and genetics between humans and baboons may increase susceptibility to interspecific infections by the same parasites and could pose a serious threat to humans and baboons (Jones-Engel et al., 2004). Niokolo-Koba National Park in Senegal, like all national parks, is a place where ecotourism is allowed and there is usually daily contact between tourists, workers, local people, and the various animals that serve as attractions. It serves as both a conservation site and a tourist attraction with interactions between some of the Park’s animals and the people living in and around the park, as well as tourists. Various studies have been carried out on the ecology of baboons in PNNKB; however, data on parasite types and prevalence in baboons within PNNKB are scarce. There is, therefore, a need to generate baseline data on parasitic infections and update the study conducted by McGrew et al. (1989) to assess the evolution of parasitism in these primates in order to effectively maintain ecosystem health and also manage the health of the baboons that are a major source of attraction in the park. Having this in mind, this study was designed to assess the prevalence and diversity of gastrointestional parasites in baboons in the Niokolo-Koba Park. Materials and MethodsStudy areaPrevious papers on baboons in PNNKB indicate a large distribution and a population size average of 269,034 individuals (Rabeil, 2015; Vale et al., 2015). Mount Assirik (12° 53′ N, 12°46′W) is an extensive volcanic hill area. It is the highest point of PNNKB with an altitude of 311 m that is only accessed by a trail. From the flattened top of the plateau, ridges and gullies radiate out, giving rise to streams that flow into the Gambia River. Our research team was based at Assirik camp where researchers usually camp with accompanying park staff. In fact, two groups of baboons frequent the area around the camp within a radius of about 4 km and frequently spend the night there. In the study area, two seasons are noted with a dry season of 9 months (October–June) and a rainy season of 3 months (July–September). The main types of vegetation that can be distinguished in the Mount Assirik area are five, including the plateaus where a carpet of grasses (Danthoniopsis tuberculata) predominates during the rainy seasons; the grassland with Andropogon sp. is the dominant grass, but there is the presence of palm trees such as Raphia sudanica and acacias; the wooded savanna is the most frequent vegetation type containing tree species (Pterocarpus erinaceus, Adansonia digitata, Ficus sp., etc.); climbing plants (Saba senegalensis, Landolphia dulcis, and Nauclea latifolia); the bamboo grove with Oxytenanthera abyssinica; and the gallery forest with the presence of Khaya senegalensis and Ceiba pentandra. Mount Assirik is frequented by scarce and emblematic species such as the West African chimpanzee (Pan troglodytes verus) and derby eland (Taurotragus derbianus). There are also other primate species that coexist with baboons in the site, including the green monkey (Cercopithecus aethiops sabaeus); the galago (Galago senegalensis), and the patas (Erythrocebus patas). In general, the fauna species frequently encountered are the warthog (Phacochoerus aethiopicus), the roan antelope (Hippotragus equinus), the hartebeest (Alcelaphus buselaphus), and the red-sided duiker (Cephalophus rufilatus). Nevertheless, the existence of some predators is noted. These are lions (Panthera leo), hyenas (Crocuta crocuta), leopards (Panthera pardus), and wild dogs (Lycaon pictus) (Lindshield et al., 2019). Data collectionBaboon feces were collected in June 2019 and analyzed between October and December 2019 (Fig. 1). During the study period, baboon’s fresh fecal samples were collected during the day. Prior to this collection, familiarization with the recognition and identification of baboon feces was done. During sampling, 50 fecal samples from 2 groups of baboons (a and b) were collected. To avoid contamination, fecal samples were collected from the center of each fecal mass, an average of 10 g of stool per sample. The samples were individually placed in sterile 50.0 ml vials and preserved in a 10% formalin solution and then the vials were stored at room temperature, as recommended by several studies (Howells et al., 2011; Kouassi et al., 2015; N’da et al., 2021), given that analysis of the samples was to be done carried out months after collection. Sample processingEach sample was processed in the laboratory using a parasite concentration method called the Ritchie method. The Ritchie method is a powerful enrichment method. It is a biphasic technique that concentrates parasitic elements by combining sedimentation using centrifugation and the removal of digestion residues by the dissolving action of diethyl ether. This method is used to diagnose helminths and protozoa (Young et al., 1979; Erdman, 1981). In addition to the above method, we conducted a second analysis of each sample by the flotation method. The principle consists of diluting the feces in a liquid supersaturated with sodium chloride so that under the action of centrifugation, the parasitic elements rise to the surface of the liquid where they can be collected. The latter is easy to use and provides a useful solution for the detection of nematodes, cestodes, and protozoa (Beugnet et al., 2004). Parasites were detected with a microscope (Leica DM500 LED) equipped with a digital camera control unit. They were identified on the basis of shape, size, color, and content. Representatives of each parasite were photographed.
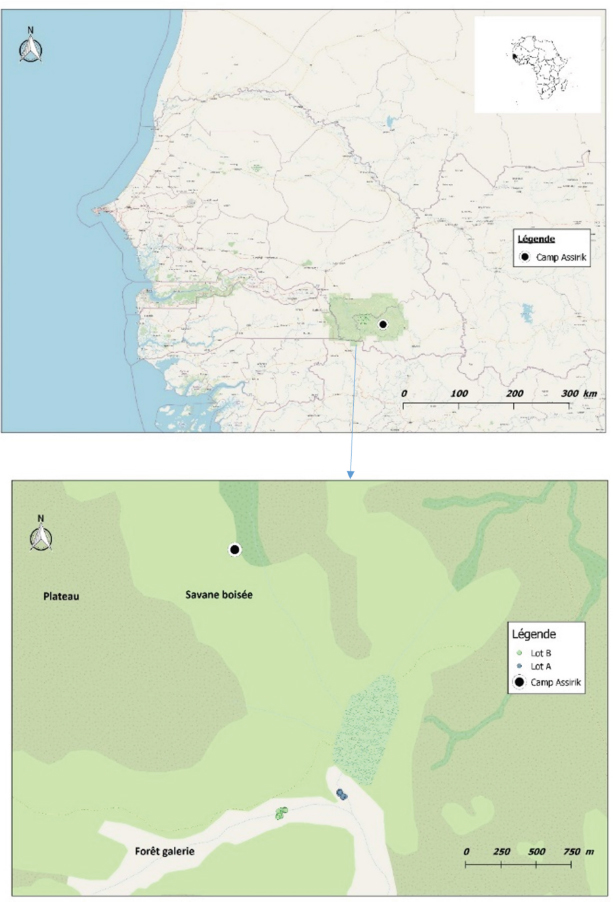
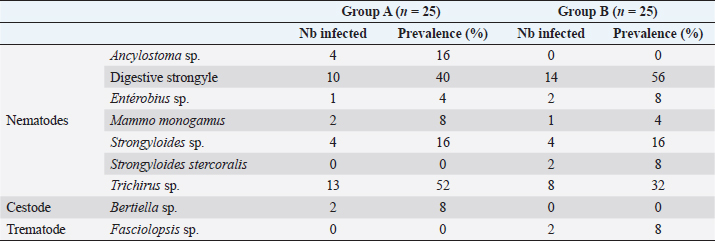
Fig. 1. Location of baboon dormitories in Assirik camp (PNNKB). Statistical analysisData from the coproscopic analysis (coproscopies and parasites species) were entered into a Microsoft® Office Excel spreadsheet under Windows 2013 and then transferred to R software version 3.4.3. to perform the prevalence calculation. Ethical approvalOur study was noninvasive. All procedures performed in this study were in accordance with the legal requirements of Senegal. ResultsIdentification of parasites and overall infestationSeven nematodes, one tapeworm, and one trematode were identified during the analysis of baboons’ feces (Fig. 2). These were Strongyloides sp., Trichirus sp., Ancylostoma sp., Mammo monogamus, Enterobius sp., S. stercoralis, a digestive strongyle, Bertiella sp (cestode), and Fasciolopsis sp. (trematode). Of the 50 samples analyzed from the baboon groups, 39 were positive for at least 1 parasite species, with a prevalence of 78%. However, of the positive samples (39), 19 were poly-infested, with a prevalence of 49%. Prevalence of parasite species in baboonsOut of nine parasites detected in all baboons, group A was infested by six nematodes (Strongyloides sp., Trichirus sp., Ancylostoma sp., M. monogamus, Enterobius sp., Strongyle digestif) and 1 cestode (Bertilla sp.). As for group B, the baboons were infested by only seven of the nine parasites, namely Strongyloides sp., Trichirus sp., M. monogamus, Enterobius sp., digestive strongyle, S., and Fasciolopsis sp. The prevalence of the different parasites found in the two groups is summarized in Table 1. DiscsussionThe results of this work provide important information on the study of baboon parasitism in PNNKB. The analyses performed revealed a fairly high prevalence (78%) of gastrointestinal parasites with the presence of nine parasitic species (seven nematodes, one trematode, and one cestode). The study conducted by McGrew et al. (1989) revealed the presence of 10 species with the existence of species of protozoan parasites that we did not find in our samples. One tapeworm (Bertiella sp.) was found in our samples, which was not the case in the study by McGrew et al. (1989). In short, we noted an evolution of parasitic species over time with the presence of species with zoonotic potential. In general, previous studies conducted on baboons report a relatively varied number of parasites; 18 parasites were found in Fongoli in Senegal (Howells et al., 2011); 13 were found in Gombe Park in Tanzania (Müller-Graf et al., 1996); 12 parasites in the central desert of Namibia (Appleton and Brain, 1995); 11 parasites were found in Kibale National Park in Uganda (Bezjian et al., 2008); and 8 parasites were found in the Shai Reserve in Ghana (Larbi et al., 2020). The variation in the number and species of parasites between study sites may be explained by the variation in ecological factors (biotic and abiotic) between different eco-geographic regions. The example of parasites pathogenic to humans, such as Entamoeba hystolitica and G. duodenalis, are destroyed at a temperature above 50 degrees. Other abiotic factors such as the physicochemical conditions of the environment and ultraviolet rays can vary the infesting power of certain parasites. According to various research studies on baboon parasitism (McGrew et al., 1989; Appleton and Brain, 1995; Müller-graf et al., 1996; Murray et al., 2000; Bezjian et al., 2008; Howells et al., 2011; Ryan et al., 2012; Larbi et al., 2020), there is an overall high prevalence, as shown in our study. The high prevalence observed in the present study could be attributed to the foraging behavior and high mobility of baboons that predispose them to frequent contact with pathogens that may be intestinal parasites disseminated in the environment. This could also be due to a more frequent human presence in and around Assirik camp, which results in baboons having more routine contact with humans during their foraging. Larbi et al. (2020) described a similar situation in Ghana. According to Hahn et al. (2003), baboons are susceptible to most human pathogens, such as parasites and diarrhea-causing bacteria; thus, baboon behavior coupled with anthropogenic changes provide opportunities for parasite exchange between baboons and with humans. This has been confirmed by the presence of Trichuris sp. in baboon feces. Trichuris sp. was the most prevalent species in our two groups (A and B). This nematode causes an identical pathology (Trichuriasis) in animals and humans. This similarity could suggest the possibility of a zoonotic transmission. It has been found in other studies including the zoo in Ibadan, Nigeria, where its prevalence was 47.2% (Adetunji, 2014); the Kibale National Park in Uganda, with a prevalence of 46% (Bezjian et al., 2008); the Gombe National Park in Tanzania, with a prevalence of 66% (Murray et al., 2000); and the Tai National Park in Côte d’Ivoire, with a prevalence of 93.02% (Kouassi et al., 2015). Strongyles come second in terms of representativeness in both groups with a nonsignificant distribution between the two groups. These parasites are frequently found in domestic animals, especially in cattle (Barré and Moutou, 1982). However, they are found in the different studies mentioned above of intestinal parasitism in baboons. Strongyloides stercoralis is encountered in many studies where it is sometimes the most encountered species (Kwigonda et al., 2015) in Kahuzi-Biega National Park, South Kivu, DR Congo. Indeed, several similar studies on primates show the presence of these zoonotic parasites in primate feces (Gillespie et al., 2010; N’da et al., 2021). Nonhuman primates are known to be the primary hosts of S. stercoralis and, in particular, Strongyloides fuelleborni. Monkeys become infected by ingestion of parasite-contaminated food or water or by skin penetration of infective third-stage larvae (Gillespie and Chapman, 2006). During massive infestation with Strongyloides, severe gastroenteritis, hepatitis, pneumonia, and myocarditis occur in both primates and humans (Toma et al., 2000). Mortality can occur if adequate treatment is not provided. Little attention has been paid to the risk of zoonotic transmission posing a public health threat (Thompson and Smit, 2011). In environments where animals and humans may interact, it is critical to understand the risk of zoonotic diseases transmission (Bowden and Drake, 2013). Changes in an anthropogenic habitat will result in frequent contact between humans and nonhuman primates, increasing the risk of diseases transmission (Nunn et al., 2016).
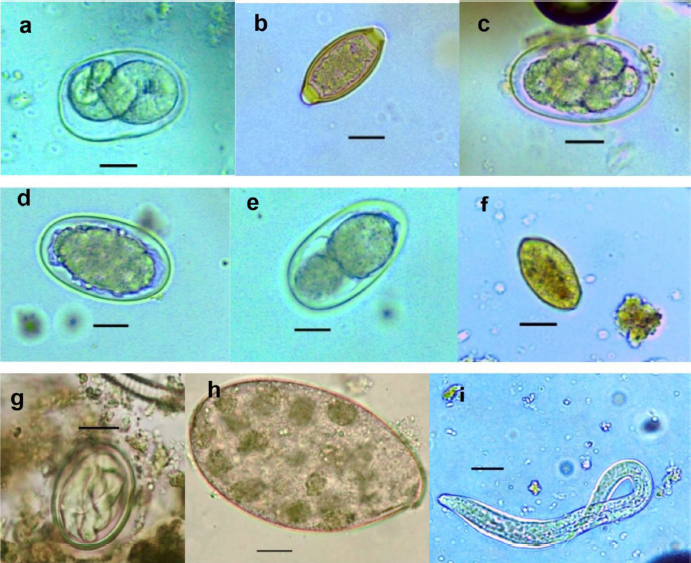
Fig. 2. (a) Strongyloides sp.; (b) Trichirus sp.; (c) Ancylostoma sp.; (d) Digestive strongle; (e) M. monogamus sp.; (f) Entérobius sp.; (g) Bertiella sp.; (h) Fasciolopsis sp.; and (i) S. stercoralis (larverhabditoide). Scale bar a–h =17.8 µm. Table 1. Prevalence (%) of gastrointestinal parasites in baboons in Niokolo-Koba Park.
Entreobius sp. and M. monogamus were found in both groups. This result suggests that these groups patronize common spaces. Enterobius sp. was found in guenons in Kibale National Park, Uganda (Bezjian et al., 2008). This result is qualified by the fact that guenons are arboreal in nature, whereas baboons are much more terrestrial. The high prevalence of Trichuris sp. found in this study is evidence of this. Mammo monogamus is an unusual parasite of baboons. It has been found in the mandrill, a cousin of the baboon, in Gabon (Allela, 2005; Setchell et al., 2007). This respiratory tract parasite is still relatively unknown and has been found in a variety of animal species, including ruminants, deer, cats, and humans (Wang, 2009). Ancylostoma sp. is a common host in the digestive tract of nonhuman primates. Its presence with significant difference between the two groups could be related to the season. Indeed, the eggs have a low resistance in a dry environment (Herbert, 2009). The parasite is also common to carnivores, notably in the spotted hyena in Ethiopia (Graber et al., 1980). It has been found in carnivores in the Hann zoological garden in Senegal (Dahourou et al., 2017) where its prevalence was higher than those found in free-ranging animals. This resistance of free-ranging animals can be explained by the development of a stronger immunity (Luffau et al., 1972). This study revealed the presence of cestodes (Bertiella sp.) and trematodes (Fasciolapsis sp.). These two groups of parasites differ from the first in that their cycles require the presence of intermediate hosts to be complete. Bertiella sp. is a typical parasite of nonhuman primates (Servián et al., 2020). The different levels of infestation of hosts are related to the interactions between parasites and hosts (Anderson and Gordon, 1982). The presence of cestode and trematode, respectively, in groups A and B characterizes this heterogeneity that could be explained by the habitat, exposure level, and physiological characteristics of the host (Bundy et al., 1987; Anderson and May, 1991; Schmid-Hempel and Koella, 1994). Nematodes are the most numerous species in the animal kingdom, according to Otero-Abad et al. (2017). This explains their relative importance in the number of parasites found (seven out of nine). Indeed, in the study by McGrew et al. (1989), the same observation was made. They found 10 species of nematodes in the 2 study sites combined, namely Mount Assirik and Gombe Park. However, by comparing their study carried out at Mount Assirik to ours, we found significantly more species of nematodes (seven species) than in their historical study (five species). The absence of protozoan parasites may be attributed to the dry conditions and the time of sampling at PNNKB (June 2019) that favor the transmission and development of nematodes more than protozoa (Bliss, 2009). It is likely that these protozoa are not able to withstand the harsh environmental conditions and may die quickly if they are not able to encyst. It may also be low because of the preservation and identification methods used, which may have killed the cysts and trophozoites (Hausfater and Watson, 1976). Intestinal parasites in baboons could be permanent with significant morbidity. However, some of the plants consumed by baboons as food may be medicinal with active ingredients against some of the parasites (Larbi et al., 2020); thus, although baboons may harbor these parasites, they may not suffer serious complications. Adult baboons use their fingers to uproot plants and feed them to juveniles in addition to what they would eat, making them more vulnerable to these soil-borne infections. In addition, susceptibility to infections is one of the costs associated with dominance (Larbi et al., 2020). In short, we have noted an evolution over time with regard to the parasitic species found in these primates. However, the presence of zoonotic parasites capable of infecting humans remains constant. This study, considered as a pilot study, provided us with results that open up new avenues of research. However, other studies will be considered in order to conduct large-scale research on baboons. ConclusionThis study identified nine species of intestinal parasites, showing diversity in baboons. The overall prevalence of 78% was high. It will also be important to educate people and tourists on standard hygiene practices in the park to prevent infections as some of the parasites revealed by the study are zoonotic or common to both humans and animals. In the current context of re-emergence of diseases, caution remains necessary. AcknowledgmentsWe thank our partners at Purdue University, West Lafayette, IN, USA for their contribution to the funding of this study. We also thank the Parasitology and Mycology Laboratory of the Inter-State School of Veterinary Science and Medicine of Dakar which allowed us to carry out parasitological analysis. Conflict of interestThe authors declare that there is no conflict of interest. ReferencesAdetunji, V.E. 2014. Prevalence of gastro-intestinal parasites in primates and their keepers from two zoological gardens in Ibadan, Nigeria. Sokoto J. Vet. Sci. 12(2), 25–30. Allela, N.L. 2005. Contribution de l’étude de l’impact de la santé des primates en aires protégées sur celles des populations humaines: examens parasitologiques chez les travailleurs du WCS et chez les mandrills (Mandrillus sphinx) du parc national de la Lopé. Thèse: Méd. Vét., Toulouse, France, p. 72. Anderson, R.M. and Gordon, D.M. 1982. Processes influencing the distribution of parasite numbers within host populations with special emphasis on parasite-induced host mortalities. Parasitology 85(2), 373–398. Anderson, R.M. and May, R.M. 1991. Infectious diseases of humans: dynamics and control. Oxford, UK: Oxford University Press, pp: 746. Appleton, C.C. and Brain, C. 1995. Gastro-intestinal parasites of Papio cynocephalusursinus living in the central Namib desert, Namibia. Afr. J. Ecol. 33(3), 257–265. Barré, N. and Moutou, F. 1982. Helminthes des animaux domestiques et sauvages de La Réunion. Inventaire et rôle pathogène. I. Mammifères. Rev. Elev. Med. Vet. Pays Trop. 35(1), 43–55. Beugnet, F., Bourdoiseau, G. and Dang, H. 2004. Abrégé de parasitologie clinique des carnivores domestiques. Volume 1. Parasitoses digestives. Kalianxis, Auxon, p. 266. Bezjian, M., Gillespie, T.R., Chapman, C.A. and Greiner, E.C. 2008. Coprologic evidence of gastrointestinal helminths of forest baboons, Papio anubis, in Kibale National Park, Uganda. J. Wildl. Dis. 44(4), 878–887. Bliss, D.H. 2009. The control of gastro-intestinal nematode parasites of hoofed wildlife in North America. Verona, WI: Technical Bulletin: MidAmerica Agricultural Research. Bowden, S.E. and Drake, J.M. 2013. Ecology of multi-host pathogens of animals. Nat. Edu. Knowl. 4(8), 5. Bundy, D.A.P., Cooper, E.S., Thompson, D.E., Anderson, R.M. and Didier J.M. 1987. Age-related prevalence and intensity of Trichuris trichiura infection in a St. Lucian community. Transac. Royal Soc. Trop. Med. Hyg. 81(1), 85–94. Dahourou, L.D., Gbati, O.B., Nacanabo, I., Diatta, C. and Pangui, L.J. 2017. Gastrointestinal parasitism in wildlife at Hann Zoological Park in Senegal. Rev. Elev. Med. Vet. Pays Trop. 70(1), 25–28. Dixon, B.R. 2021. Giardia duodenalis in humans and animals–transmission and disease. Res. Vet. Sci. 135, 283–289. Erdman, D.D. 1981. Clinical comparison of ethyl acetate anddiethyl ether in the formalin-ether sedimentation technique. J. Clin. Microbiol. 14(5), 483–485. Gillespie, T.R. and Chapman, C.A 2006. Prediction of parasite infection dynamics in primate metapopulations based on attributes of forest fragmentation. Biol. Conserv. 20(2), 441–448. Gillespie, T.R., Lonsdorf, E.V., Canfield, E.P., Meyer, D.J., Nadler, Y., Raphael, J, Pusey, A.E., Pond, J., Pauley, J., Mlengeya, T. and Travis, D.A 2010. Demographic and ecological effects on patterns of parasitism in eastern chimpanzees (Pan troglodytes schweinfurthii) in Gombe National Park, Tanzania. Am. J. Phys. Anthropol. 143(4), 534–544. Graber, M., Blanc, P. and Delavenay, R. 1980. Helminthes des animaux sauvages d’Ethiopie. I. Mammifères. Rev. Elev. Med. Vet. Pays Trop. 33(2), 143–158. Ghosh, S., Padalia, J. and Moonah, S. 2019. Tissue destruction caused by Entamoeba histolytica parasite: cell death, inflammation, invasion, and the gut microbiome. Curr. Clin. Microbiol. Rep. 6(1), 51–57. Hahn, N.E., Proulx, D., Muruthi, P.M., Alberts, S. and Altmann, J. 2003. Gastrointestinal parasites in free-ranging Kenyan baboons (Papio cynocephalus and P. anubis). Int. J. Primatol. 24(2), 271–279. Hausfater, G. and Watson, D.F. 1976. Social and reproductive correlates of parasite ova emissions by baboons. Nature 262(5570), 688–689. Herbert, A. 2009. Contribution à l’étude du parasitisme chez le mandrill au Gabon. Doctoral dissertation, Toulouse, France, p. 72. Howells, M.E., Pruetz, J. and Gillespie T.R. 2011. Patterns of gastro-intestinal parasites and commensals as an index of population and ecosystem health: the case of sympatric Western chimpanzees (Pan troglodytes vs.) and Guinea baboons (Papio hamadryas papio) at Fongoli, Senegal. Am. J. Primatol. 73(2), 173–179. Jones-Engel, L., Engel, G.A., Schillaci, M.A., Froehlich, J., Paputungan, U. and Kyes, R.C. 2004. Prevalence of enteric parasites in pet macaques in Sulawesi, Indonesia. Am. J. Primatol. 62(2), 71–82. Kouassi, R., McGraw SW., Kouassi P., Boubacar, A., Brunet, J., Pesson, B., Banfoh, B., N’goran, E.K. and Candolfi, E. 2015. Diversity and prevalence of gastrointestinal parasites in seven non-human primates of the Taï National Park, Côte d’Ivoire. Parasite 22(1), 13. Kwigonda, J.F., Mushobekwa, J.K. and Bisimwa, A. 2015. Problématique des incursions de babouins du Parc National de Kahuzi-Biega (PNKB) vers les sites agricoles du groupement de Bugorhe, Sud-Kivu, RD Congo [Problematic of Baboons incursions of KahuziBiega National Park (KBNP) to agricultural sites of Bugorhe’s village, Souf-Kivu, DRC]. Int. J. Innov. Sci. Res. 13(1), 83–88. Larbi, J.A., Akyeampong, S., Abubakari, A., Offei Addo, S., Okoto, D. and Hanson, H. 2020. Zoonotic gastrointestinal parasites of baboons (Papioanubis) in the Shai Hill Reserve in Ghana. BioMed. Res. Int. 2020, 6; doi:10.1155/2020/ Legesse, M. and Erko, B. 2004. Zoonotic intestinal parasites in Papioanubis (baboon) and Cercopithecus aethiops (vervet) from four localities in Ethiopia. Acta Tropica 90(3), 231–236. Lindshield, S., Bogart, S.L., Gueye, M., Ndiaye, P.I. and Pruetz, J.D. 2019. Informing protection efforts for critically endangered chimpanzees (Pan troglodytes vs.) and sympatric mammals amidst rapid growth of extractive industries in Senegal. Folia Primatol. 90, 124–136. Luffau, G., Aynaud, J.M., Metzger, J.J. and Paraf, A. 1972. Le rôle de la tolérance immunitaire dans la pathogénie des infections virales et des infestations parasitaires. Ann. Res. Vét. 3(2), 251–272. McGrew, W.C., Tutin, C.E.G., Collins D.A. and File, S.K. 1989. Intestinal parasites of sympatric Pan troglodytes and Papio spp. at two sites: Gombe (Tanzania) and Mt. Assirik (Senegal). Am. J. Primatol. 17, 147–155. Messager, A. 2018. Etude épidémiologique du parasitisme influençant la production de cachemire à Bayankhongor (Mongolie) et propositions de solutions adaptées dans le cadre d’une production durable. Doctoral dissertation, p. 118. Müller-Graf, C.D.M., Collins, D.A. and Woolhouse, M.E.J. 1996. Intestinal parasite burden in five troops of olive baboons (Papio cynocephalus anubis) in Gombe Stream National Park, Tanzania. Parasitology 112(5), 489–497. Murray, S., Stem C., Boudreau, B. and Goodall, J. 2000. Intestinal parasites of baboons (Papio cynocephalus anubis) and chimpanzees (Pan troglodytes) in Gombe National Park. J. Zoo Wildl. Med. 31(2), 176–178. N’da, K.M., Dahourou, L.D., Gbati, O.B. and Alambedji, R.B. 2021. Diversity and prevalence of gastrointestinal parasites with zoonotic potential of Green Monkeys in Bandia Reserve in Senegal. Int. J. One Health 7(1), 65–70. Nunn, C.L., Gillespie, T.R., Wich, S. and Marshall A. 2016. Infectious disease and primate conservation. In: An introduction to primate conservation, pp: 157–174. Ocaido, M., Dranzoa, C. and Chel, P. 2003. Gastrointestinal parasites of baboons (Papio anubis) interacting with humans in West Bugwe Forest Reserve, Uganda. Afr. J. Ecol. 41(4), 356–359. Otero-Abad, B., Rüegg, S.R., Hegglin, D., Deplazes, P. and Torgerson, P.R. 2017. Mathematical modelling of Echinococcus multilocularis abundance in foxes in Zurich, Switzerland. Parasit. Vect. 10(1), 1–12. Rabeil, T. 2015. Inventaire de la grande faune du Parc National du Niokolo Koba (PNNK). Sénégal, West Bengal: UNESCO-MEDD, p. 39. Ryan, S.J., Brashares, J.S., Walsh, C., Milbers, K., Kilroy, C. and Chapman, C.A. 2012. A survey of gastrointestinal parasites of olive baboons (Papio anubis) in human settlement areas of Mole National Park, Ghana. J. Parasitol. 98(4), 885–888. Schmid-hempel, P. and Koella J.C. 1994. Variability and its implications for host-parasite interactions. Parasitol. Today 10(3), 98–100. Servián, A., Zonta, M.L., Cociancic, P., Falcone A., Ruybal, P., Capasso, S. and Navone, G.T., 2020. Morphological and molecular characterization of Bertiella sp. (Cestoda, Anoplocephalidae) infection in a human and howler monkeys in Argentina. Parasitol. Res. 19(4), 1291–1300. Setchell, J.M., Bedjabaga, I.B., Goossens, B.P.R., Wickings, E.J. and Knapp, L.A. 2007. Parasite prevalence, abundance, and diversity in a semi-free-ranging colony of Mandrillus sphinx. Int. J. Primatol. 28(6), 1345–1362. Thompson, R.C.A. and Smit A. 2011. Zoonotic enteric protozoa. Vet. Parasitol. 182(1), 70–78. Toma, H., Sato, Y., Shiroma, Y., Kobayashi, J., Shimabukuro, I. and Takara M. 2000. Comparative studies on the efficacity of three anthelminthics on treatment of human strongyloidiasis in Okinawa, Japan. South East Asian J. Trop. Med. Public Health 31(1), 147–151. Vale, C.G., Ferreira da Silva, M.J., Campos, J.C., Torres, J. and Brito, J.C. 2015. Applying species distribution modelling to the conservation of an ecologically plastic species (Papio papio) across biogeographic regions in West Africa. J. Nature Conserv. 27, 26–36. Wang, A. 2009. Parasite project Mammomonogamus. [in line] disponible sur. Available via https://web.stanford.edu/group/parasites/ParaSites2009/AmyWang_Mammomonogamus/AmyWang_mammomonogamus.htm (Accessed 4 June 2021). WHO Expert Committee. 1987. Public health significance of intestinal parasitic infections. Geneva, Switzerland: WHO 65(5), 575. Young, K.H., Bullock S.L., Melvin D.M. and Spruill C.L. 1979. Ethyl acetate as a substitute for diethyl ether in the formalin-ether sedimentation technique. J. Clin. Microbiol. 10(6), 852–885. | ||
| How to Cite this Article |
| Pubmed Style Nda KM, Dahourou LD, Ndiaye PI, Lindshield S, Gbati OB, Traore A. Gastrointestinal parasites of baboons (Papio papio) in NiokoloKoba National Park, Senegal. Open Vet. J.. 2022; 12(4): 481-488. doi:10.5455/OVJ.2022.v12.i4.9 Web Style Nda KM, Dahourou LD, Ndiaye PI, Lindshield S, Gbati OB, Traore A. Gastrointestinal parasites of baboons (Papio papio) in NiokoloKoba National Park, Senegal. https://www.openveterinaryjournal.com/?mno=30816 [Access: January 25, 2026]. doi:10.5455/OVJ.2022.v12.i4.9 AMA (American Medical Association) Style Nda KM, Dahourou LD, Ndiaye PI, Lindshield S, Gbati OB, Traore A. Gastrointestinal parasites of baboons (Papio papio) in NiokoloKoba National Park, Senegal. Open Vet. J.. 2022; 12(4): 481-488. doi:10.5455/OVJ.2022.v12.i4.9 Vancouver/ICMJE Style Nda KM, Dahourou LD, Ndiaye PI, Lindshield S, Gbati OB, Traore A. Gastrointestinal parasites of baboons (Papio papio) in NiokoloKoba National Park, Senegal. Open Vet. J.. (2022), [cited January 25, 2026]; 12(4): 481-488. doi:10.5455/OVJ.2022.v12.i4.9 Harvard Style Nda, K. M., Dahourou, . L. D., Ndiaye, . P. I., Lindshield, . S., Gbati, . O. B. & Traore, . A. (2022) Gastrointestinal parasites of baboons (Papio papio) in NiokoloKoba National Park, Senegal. Open Vet. J., 12 (4), 481-488. doi:10.5455/OVJ.2022.v12.i4.9 Turabian Style Nda, Kacou Martial, Laiban Dieudonn Dahourou, Papa Ibnou Ndiaye, Stacy Lindshield, Oubri Bassa Gbati, and Amadou Traore. 2022. Gastrointestinal parasites of baboons (Papio papio) in NiokoloKoba National Park, Senegal. Open Veterinary Journal, 12 (4), 481-488. doi:10.5455/OVJ.2022.v12.i4.9 Chicago Style Nda, Kacou Martial, Laiban Dieudonn Dahourou, Papa Ibnou Ndiaye, Stacy Lindshield, Oubri Bassa Gbati, and Amadou Traore. "Gastrointestinal parasites of baboons (Papio papio) in NiokoloKoba National Park, Senegal." Open Veterinary Journal 12 (2022), 481-488. doi:10.5455/OVJ.2022.v12.i4.9 MLA (The Modern Language Association) Style Nda, Kacou Martial, Laiban Dieudonn Dahourou, Papa Ibnou Ndiaye, Stacy Lindshield, Oubri Bassa Gbati, and Amadou Traore. "Gastrointestinal parasites of baboons (Papio papio) in NiokoloKoba National Park, Senegal." Open Veterinary Journal 12.4 (2022), 481-488. Print. doi:10.5455/OVJ.2022.v12.i4.9 APA (American Psychological Association) Style Nda, K. M., Dahourou, . L. D., Ndiaye, . P. I., Lindshield, . S., Gbati, . O. B. & Traore, . A. (2022) Gastrointestinal parasites of baboons (Papio papio) in NiokoloKoba National Park, Senegal. Open Veterinary Journal, 12 (4), 481-488. doi:10.5455/OVJ.2022.v12.i4.9 |