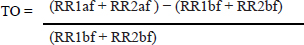| Original Article | ||
Open Vet. J.. 2021; 11(4): 635-644 Open Veterinary Journal, (2021), Vol. 11(4): 635–644 Original Research Assessment of heart rate turbulence in dogs with myxomatous mitral valve diseaseJulio P. dos Santos*, Stephany B. Lucina, Bruna N. da Costa, Karla L. C. Olaguivel, Giovana L. R. Tuleski and Marlos G. SousaLaboratory of Comparative Cardiology, Department of Veterinary Medicine, Federal University of Paraná (UFPR), Curitiba, Brazil *Corresponding Author: Julio P. dos Santos. Laboratory of Comparative Cardiology, Department of Veterinary Medicine, Federal University of Paraná (UFPR), Curitiba, Brazil. Email: juliopsvet [at] gmail.com Submitted: 05/04/2021 Accepted: 21/10/2021 Published: 13/11/2021 © 2021 Open Veterinary Journal
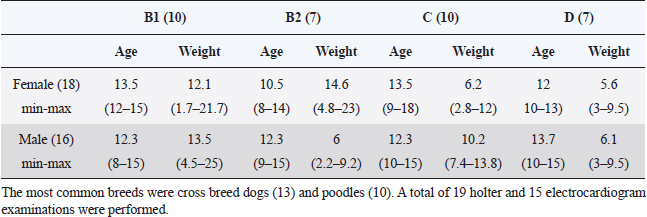
AbstractBackground: Myxomatous mitral valve degeneration (MMVD) is the most common heart disease affecting small dogs, it reduces cardiac output resulting in compensatory adaptation of the autonomic nervous system. Chronically, it leads to reduced heart rate variability (HRV) which is an accurate marker for autonomic balance. More than two decades ago in human medicine an indicator of autonomic balance that happens after a premature ventricular beat, it was described as heart rate turbulence (HRT). In humans with ischemic heart disease, the absence of HRT has proven to be a more accurate and an independent indicator of mortality than known HRV parameters. Currently, there are very few studies of HRT in dogs and it is still not tested in small dogs within different stages of myxomatous mitral valve disease. Aim: The aim of this study was to assess the HRT indicators, onset and slope, in small dogs with myxomatous mitral valve disease and to statistically test it. Methods: Dogs under 25 kg had electrocardiogram and echocardiography performed and, in some patients, holter monitoring was carried out. Data were divided into groups B1, B2, C, and D for mean comparison with analysis of variance and Tukey test. In addition, receiver operating characteristic (ROC) curve for differentiating among symptomatic and asymptomatic dogs and for differentiating between remodeled and non-remodeled hearts. The Pearson was executed after correlations of turbulence onset (TO) and turbulence slope (TS) with commonly used echocardiographic parameters. Results: Variance analyses held significant differences in TO and TS between stages B1 from stages C and D, while B2 held similarity to the other groups. In the receiver operating curve was found a very good area under the curve for differentiating among symptomatic and asymptomatic dogs and remodeled and non-remodeled dogs. Few echocardiography parameters held weak correlation with TO while others held weak to moderate correlation with TS. Conclusion: In dogs with MMVD and without other diseases, HRT is a feasible indicator for autonomic balance. Our result suggests HRT changes as the MMVD progresses and congestive heart failure is present. More studies with HRT are needed. The number of ventricular premature contractions (VPCs) may be the strongest limitation for the technique. Keywords: Baroreflex, Autonomic balance, Heart rate variability, Turbulence slope, Turbulence onset. IntroductionIn human medicine, a baroreflex indicator is heart rate turbulence (HRT). This demonstrates the response of the cardiac cycle after a premature ventricular beat interrupts sinus rhythm. This non-invasive method stratifies the risk of death and proved to be a strong and independent indicator in man with results better than heart rate variability (HRV) (Schmidt et al., 1999). The HRT is considered to be a biphasic indicator, starting with the turbulence onset (TO), defined as the initial or the acceleration phase and the turbulence slope (TS), considered the terminal or deceleration phase. Intrapatient variability may occur depending on coupling interval time, and heart rate (Bauer et al., 2008). In people with mitral valve prolapse HRT was statistically different from normal subjects, while HRV showed no difference (Gunduz et al., 2006). When divided by the New York Heart Association scale classes I and II had a significantly higher HRT slope than patients in class III (Davies et al., 2001). In dogs myxomatous mitral valve degeneration (MMVD) also known as endocardiosis accounts for 75% of all cardiovascular diseases in dogs under 25 kg and is the heart disease with the highest morbidity and mortality. Clinical signs are generally seen >7 years of age. The mitral degeneration leads to regurgitation and reduced cardiac pumping ability causing volume overload of the left atrium and ventricle (Borgarelli et al., 2008; Fox, 2012). The objective of the present study was to assess and compared the HRT in dogs with myxomatous mitral valve disease at stages B1, B2, C, and D, to calculate it sensitivity and sensibility through receiver operator characteristic (ROC) analyses and to check for correlations with commonly used echocardiographic parameters. Materials and MethodsThe study was conducted at the Veterinary Hospital of Universidade Federal do Paraná. The data collection was designed to gather only information of male and female dogs under 25 kg presenting a mitral valve regurgitation due to mitral valve myxomatous degeneration in stages B1, B2, C, and D. The retrospective part of the study was done with data of 15 dogs while for the prospective analysis 18 dogs were accepted. The total number of dogs enrolled in groups B1, B2, C and D were 10, 7, 10 and 7 respectively, in a total of 34 subjects, 18 females and 16 males. The most common breeds were cross breed dogs (13% or 31%) and poodles (10% or 24%). A total of 19 holter and 15 electrocardiogram examinations were performed (Table 1). For all patients, electrocardiography, echocardiography, creatinine were available, some patients also had exams of seric cACTH or T4, those with diagnosed hyperadrenocorticism or hypothyroidism were not accepted. Retrospective patients had no holter data available, thus only their electrocardiography record was used for the analyses and only prospective patients had holter carried out. Animals in stages B2, C, and D were medicated according to American college of veterinary internal medicine (ACVIM) guidelines (Keene et al., 2019). If the prospective dogs met all the inclusion criteria the owners were invited to participate in the study by 24 hours holter monitoring of the dog. In the electrocardiography and holter if a sinus rhythm, sinus arrhythmia or sinus tachycardia was the main rhythm and at least one ventricular premature contraction (VPC) was present the patient was accepted for HRT calculation. Only dogs with maximal creatinine seric level of 1.4 mg/dl were considered having normal renal function and thus accepted (IRIS, 2019). Not all patients were tested but patients showing clinical signs of hyperadrenocorticism were assessed with seric cACTH, dexamethasone test and ultrasound as suggested by Behrend et al. (2013). Patients with clinical signs of hypothyroidism had the seric thyrotropin (TSH) and serum-free thyroxine (T4) levels assessed. The seric T4 level was assessed by dialysis method. The exclusion criteria were the inexistence of VPCs on dogs’ electrocardiography or holter recordings; interpolated VPCs were not accepted. Additionally, if the main rhythm was sustained atrial fibrillation, sustained ventricular tachycardia, sustained bigeminy, or trigeminy the dog was excluded. Dogs in groups C and D were not accepted if they were in decompensated heart failure (HF) and showing clinical signs of congestive heart failure (CHF), e.g., dyspnea or pulmonary edema. Pulmonary edema was assessed with thoracic radiographs. On the echocardiography, patients were excluded if they had severe tricuspid valve insufficiency, pulmonary hypertension greater than 75 mmHg, or any other congenital or acquired heart disease. If the dog had seric levels of creatinine, TSH and T4 out of the normal reference range or if had a positive diagnosis for hyperadrenocorticism it was excluded. EchocardiographyThe echocardiography was done by a proficient cardiologist. At first, the dog’s fur was clipped, the dog was gently held in lateral recumbency and gel applied. The right parasternal view was used to assess the size of the left ventricle and left atrium to aorta ratio (LA/Ao). To evaluate the left ventricle, the M-mode was used to measure the chamber at the point between the papillary muscles in systole and diastole, the LA/Ao was assessed through the Swedish method. The LA/Ao ratio was assessed measuring the diameter of both structures at the bidimensional view, right after the aortic valve closure. Table 1. Table containing the study demography, groups were divided according to their MMVD stage. The table contains the gender, the average, minimum and maximum age and weight of the dogs included in the study.
The dogs were classified according to the ACVIM guidelines (2019), with B1 class having mitral valve insufficiency without left atrium remodelment. The main criteria to classify B2 patients was the finding of mitral valve insufficiency and remodeled left heart, ventricle or atria, without clinical signs. Remodeled left heart was defined as LA/Ao ratio equal or greater than 1.6 or the left ventricle internal diameter indexed by the body surface area (LVDNN) greater than 1.7. The normalization formula used for diastole was the following: Left ventricular diastolic diameter/body weight 0.295 Dogs in stage C are those with remodeled hearts with past or current clinical signs like cough, dyspnea or weakness caused by the MMVD. Class D were patients that even under therapy persistently show clinical signs of MMVD. In the same recumbency, the pulmonary valve was assessed through color doppler. After that the patients were positioned in right recumbency for the left apical 4-chamber view. In this recumbency, the color doppler was used to assess the degree valve insufficiency for the mitral, aortic, and tricuspid valves. The size of the mitral valve regurgitant jet was compared to the atrial size. The tricuspid valve was assessed via the left apical 4-chamber view optimized for the right heart. If tricuspid valve insufficiency was found in the color doppler then the continuous wave doppler was used to estimate the pulmonary artery pressure. The dog was included in the study only if no other cardiac diseases, congenital, or acquired were found. When tricuspid regurgitation was present without pulmonary valve stenosis, the pulmonary arterial pressure was estimated with the continuous doppler over the tricuspid regurgitant jet, dogs were not accepted if their estimated pulmonary arterial pressure was over 75 mmHg. Electrocardiography/holterThe electrocardiograms were recorded and holter performed by veterinary cardiologists. Human medicine articles presenting this technique recommend averaging the HRT of five or more different VPCS of the same patient (Bauer et al., 2008). A 3-minute electrocardiogram and a 24-hour holter were done in the prospective patients. Not all patients had five or more VPCs, so when a single VPC or more were available within a sinus rhythm, sinus arrhythmia or sinus tachycardia, up to five VPCs were used for the HRT calculation. After that, HRT values were averaged. The VPCs were chosen based on the coupling interval, excluding VPCs with coupling intervals shorter than 280 ms. The sum of the VPC compensatory pause plus the first respiratory rate (RR) interval had to be longer than the two RR intervals before the coupling interval with the maximum of 2,000 ms. The TO was manually calculated as described by Schmidt et al. (1999), as the difference between the mean of the first two sinus RR intervals after a VPC and last two sinus RR intervals before the VPC divided by the mean of the two sinus RR intervals before the VPC. As described by the following equation:
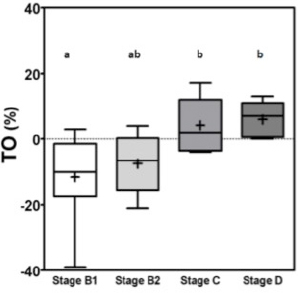
RR1af: First R-R interval after the premature ventricular contraction. RR2af: Second R-R interval after the premature ventricular contraction. RR1bf: First R-R interval before the premature ventricular contraction. RR2bf: Second R-R interval before the premature ventricular contraction. The coupling interval and the compensatory pause were considered to be point zero (0) for the calculations. The final TO result is shown as %. The TS was calculated as suggested by Schmidt et al. (1999). The R-R intervals after the VPC, were analyzed in groups of five, until the 20th R-R interval using linear regression. Then the steepest regression line found in the results was chosen as TS value. TS was expressed as milliseconds per RR (ms/RR). For statistical analyses, patients were classified according to ACVIM Consensus definitions (Keene et al., 2019) where stages B1 and B2 were classified as asymptomatic and patients stage C and D were classified as symptomatic. All data were considered parametric by the Kolmogorov–Smirnov test and the variance comparison between the groups were calculated by analysis of variance test followed by Tukey test, in these p was considered significant if less than 0.05 (p ≤ 0.05). The sensitivity and specificity were calculated by the (ROC) curve for differentiating stage B1 + B2 (non-symptomatic) from stages C + D (symptomatic) and for differentiating B1 dogs (non-remodeled hearts) from B2 + C + D dogs (remodeled hearts). The Pearson correlation test (R) was calculated after correlations between TO and TS and commonly used echocardiographic parameters, where p was considered significant if less than 0.05 (p ≤ 0.05). ResultsThe variance analyses results for TO and TS are illustrated in Figures 1 and 2. For TO, the results of stage B1 were statistically different from stages C and D, showing that stage B1 had a different response after the VPC. Stage B2 results were similar to results of stages B1, C and D. The TO means ± standard deviations (SD) for groups B1, B2, C and D are expressed in percentage (%) and were respectively −11 ± 12.5a, −7.5 ± 9.0ab, 4.2 ± 8.2b, 6.0 ± 5.5b, with p ≤ 0.005, different letters showing different groups and same letters indicating similarity. The stage B1 had its minimal value negative and close to −40% of previous R-R intervals.
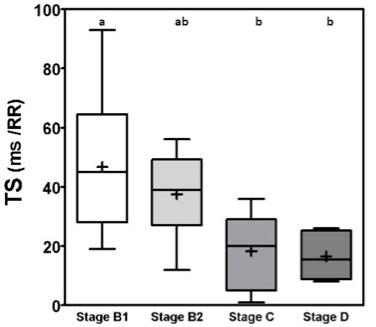
Fig. 1. Box plot graphic for TO of dogs with different stages of MMVD displaying the mean, SD and amplitude values. Stage B1 mean was statistically different from stages C and D means. Stage B2 mean was similar to stages B1, C, and D means. The mean ± SD for stages B1, B2, C, and D for TO were, respectively, −11 ± 12.5a, −7.5 ± 9.0ab, 4.2 ± 8.2b, 6.0 ± 5.5b. Different letters indicate statistically significant differences between groups ( p=0.005). TO, Turbulence onset; SD, Standard deviation. Same letters indicate similarity between groups. For TS the mean ± SD for groups B1, B2, C and D, in ms/RR, were respectively 46.8 ± 23.2a, 37.5 ± 15ab, 18.3 ± 13.5b, 16.5 ± 7.5b, p=0.0029, with different letters indicating statistically significant differences between groups (p ≤ 0.005). For both indicators stage B1 had the widest dispersion overall stages while stage D had the smallest dispersion.
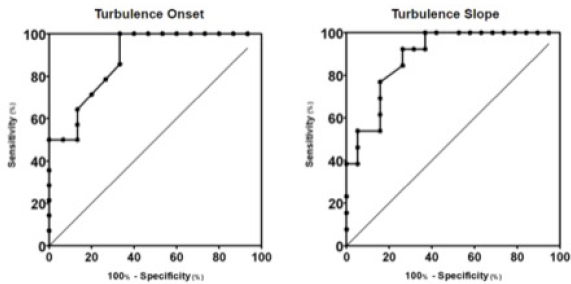
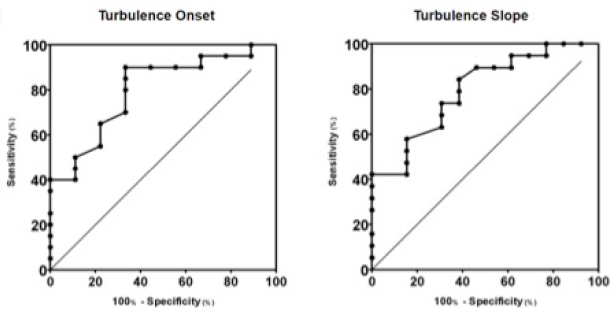
Fig. 2. Box plot graphic for TS of dogs with different stages of MMVD displaying the mean, SD and amplitude values. Stage B1 mean was statistically different from stages C and D means. Stage B2 mean was similar to stages B1, C, and D means. The mean ± SD for stages B1, B2, C, and D for TS were, respectively, 46.8 ± 23.2a, 37.5 ± 15ab, 18.3 ± 13.5b, 16.5 ± 7.5b, p=0.0029. TS, Turbulence slope; SD, Standard deviation. Same letters indicate similarity between groups. The ROC curves are plotted together in Figures 3 and 4, where we can compare the area under the curve (AUC) of TO and TS for different testing. In Figure 3, we can evaluate ROC curves for differentiating stages B1 and B2 from stages C and D, or asymptomatic dogs versus symptomatic dogs. In this interrogation, TO had an AUC (mean ± SD, 95% CI) of 0.883 ± 0.060, 0.764:1.00, while TS had an AUC (mean ± SD, 95% CI) of 0.890 ± 0.055, 0.782:0.999.
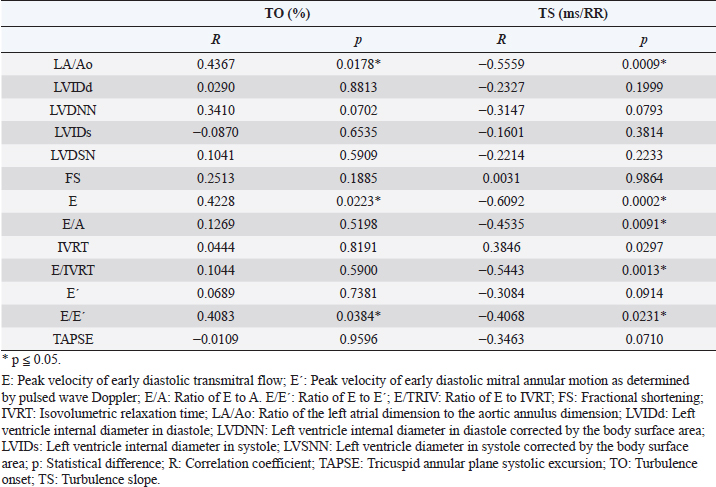
Fig. 3. ROC curves of HRT for differentiating between stages B1 + B2 and C + D, asymptomatic dogs versus symptomatic dogs. In this interrogation, TO had an AUC (mean ± SD, 95% CI) of 0.883 ± 0.060, 0.764:1.00, while TS had an AUC (mean ± SD, 95% CI) of 0.890 ± 0.055, 0.782:0.999. In Figure 4, we can evaluate ROC curves for differentiating stage B1 from stages B2, C, and D, or dogs with non-remodeled hearts versus dogs with remodeled hearts. For this purpose, TO had an AUC (mean ± SD, 95% CI) of 0.800 ± 0.087, 0.628:0.971, while TS had an AUC (mean ± SD, 95% CI) of 0.795 ± 0.079, 0.640:0.950. When compared with the echocardiography exam, the values of TO and TS also showed moderate-to-weak correlation with commonly used parameters (statistical significance was set at p ≤ 0.05). The results are shown in Table 2. The results of Pearson analyses are plotted in Table 2, there was a weak correlation of TO and LA/Ao, the maximal transmitral E wave velocity and the E/E´ ratio. The TS showed a weak negative correlation with the E/A ratio, E/E´ ratio, while moderate negative correlation was found with E/isovolumetric relaxation time (IVRT), maximal E wave velocity and the left atrium/aorta ratio. Weak positive correlations were found between TS and the IVRT, with p < 0.05. DiscussionThe main hemodynamic effect of MMVD is the regurgitant orifice that leads to a regurgitant volume and reduced cardiac output (Öztürk et al., 2016). The overload consequences come as atrial and/or ventricle enlargement by eccentric hypertrophy and in some patients have diastolic or systolic dysfunction (Rasmussen et al., 2012; Bruce et al., 2019). The autonomic system compensatory responses to HF are the reduction of parasympathetic portion response and increase in sympathetic activity associated with high serum norepinephrine (NE) concentrations (Öztürk et al., 2016). The effects of sympathetic stimulus on the circulatory system are the increase in heart rate, contractility, and cardiac relaxation, while on the blood vessels the venous capacitance reduces and in peripheral resistance increases (Lymperopoulos et al., 2013). One test used in human medicine to assess the levels of autonomic response is the HRT (Schmidt et al., 1999). In this study, we tried to assess the autonomic response of small dogs diagnosed with MMVD through HRT indicators, TO and TS, using a holter monitor and electrocardiographic records. Our results show that TO had a variation within the groups, as early stages had mean value at the negative field and with broader amplitude, different than symptomatic stages. Stage B1 mean value was statistically different from stages C and D means, it had the widest dispersion overall and seems to have a negative skew. Stage B2 showed similarity to the other stages. Stage D also had the smallest dispersion among the groups. These findings suggest that the initial acceleration phase of the turbulence, TO, is present in a large portion of early patients, mainly in stage B1, and that the autonomic response diminishes as CHF appears in chronic stages of MMVD. Table 2. Correlation between heart rate turbulence of dogs with MMVD and echocardiographic parameters.
Fig. 4. ROC curves of HRT for differentiating between stages B1 and B2 + C + D, or dogs with non-remodeled hearts versus dogs with remodeled hearts. For this purpose, TO had an AUC (mean ± SD, 95% CI) of 0.800 ± 0.087, 0.628:0.971, while TS had an AUC (mean ± SD, 95% CI) of 0.795 ± 0.079, 0.640:0.950. In the HRT early phase, the heart rate acceleration is associated with parasympathetic withdrawal in response to less vagal stimulus due to VPC ineffective blood ejection (Wichterle et al., 2002). The correlation between TO and baroreflex sensitivity was lost only after combined autonomic blockade with atropine, suggesting that, not only parasympathetic activity but also the sympathetic nervous system might influence modulation of TO (Lin et al., 2002; Marine et al., 2002). The HR acceleration seems to be linked to the vagal activity directly over the sinus node (Marine et al., 2002), and it can be linked to the spontaneous baroreflex sensitivity (Lin et al., 2002). There are evidence that beta-blocker do not reduce TO value (Marine et al., 2002). The results of TS in this study shows that the mean ± SD in stage B1 is different than in stages C and D, as well as the amplitude of TS which was greater in stage B1 than in stages C and D. The TS of stage B2 was not different to B1, C, and D. These findings suggest that the baroreflex response is present in the early stages of the disease as seen in B1 group, and shows that this response significantly decreases in latter stages of the disease, represented by stages C and D, as HF worsens due to MMVD and compensatory response is necessary. The bigger the TS the greater is heart rate deceleration, this reflects the intensity of autonomic response. In higid humans, the late phase of HRT, so called TS, is known for decrease of heart rate and return of the frequency to levels previous to the VPC (Wichterle et al., 2002). The baroreflex sensitivity is responsible for this autonomic response that has both sympathetic and parasympathetic components involved (Bauer et al., 2008). There is evidence that in humans’ intravenous atropine results in reduction of TS. Humans under B-blockers also showed reduction of both parameters. TS was abnormal in all patients after receiving atropine (Marine et al., 2002). The correlation between TS and baroreflex sensitivity was lost after selective parasympathetic and combined autonomic blockade, with atropine and atropine + esmolol. Esmolol did not influence the TS. It was seen that the maintenance of normal HRT was vagally dependent; and that both TS and TO components of HRT were closely correlated with spontaneous baroreflex sensitivity (Lin et al., 2002). The values of TO and TS in the B1 group were considered statistically different from groups C and D, while group B2 had similar values to group B1 and groups C and D, making group B2 impossible to differentiate from the others. Results from groups C and D were also statistically similar. In group B1, the TO response had a large dispersion from negative to positive, but the mean value was negative, as seen in the B2 group. In groups C and D the means were above the zero line. Of note is that the amplitude for both TO and TS tended to be smaller as disease progressed. Our results suggest that the mean TO and TS values tend to be higher as the patient’s cardiac condition worsens. The results in this study shows that as the MMVD progresses the dog becomes symptomatic and supports that the vagal response is impaired in dogs with chronic HF due to MMVD. In one study showing a time-domain analysis after 1 minute of ocular compression in dogs with MMVD, revealed that for every index, the symptomatic group presented significantly lower values than control and the non-remodeled groups. After ocular compression, non-remodeled dogs significantly increased SDNN (SD of R-R intervals), root mean square of SD of R-R intervals (RMSSD) and Vasovagal Tonus Index (VVTI), whereas healthy controls demonstrated an increase in VVTI only (Brüler et al., 2018). This comes in accordance with the present study suggesting that dogs with heart disease but no-remodeled heart have some degree of parasympathetic response present. The diminished response in symptomatic dogs shows that the parasympathetic response in patients with remodeled hearts is impaired as it happens in humans with CHF (Eckberg et al., 1971). Also humans with untreated mitral regurgitation (MR) also present worse baroreflex when compared to treated patients (Öztürk et al., 2016). This supports our findings that dogs with heart disease in CHF stage, represented by stages C and D, have diminished autonomic response than dogs in non-congestive stages. In our study, there was no statistical difference between stage B2 and other stages for both TO and TS indicators. This agrees with another study showing that asymptomatic dogs with remodeled hearts have lower SDNN, RMSSD, and VVTI when compared to dogs with non-remodeled hearts. After ocular compression, there was no significant variation in these parameters in remodeled groups (Brüler et al., 2018). This supports the theory that autonomic imbalance precedes clinical evidence of heart disease (TFESCNASPE (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology), 1996). In dogs with dilated cardiomyopathy, significantly different results for TO and TS were found when comparing them to normal dogs, additionally dogs that survived more than 30 days after diagnosis had TO and TS different from dogs that did not survive (Noszczyk-Nowak, 2012a). Similar results were found in Doberman pinschers, where the normal dogs had the TO mean value significantly more negative than dogs with dilated cardiomyopathy (DCM) and DCM + CHF dogs. The TS for DCM and DCM + CHF dogs was significantly different than normal dogs (Harris et al., 2017). Furthermore, in the present study plus two other studies investigating HRT in dogs, the results show that the SD of the HRT parameters reduced as CHF progressed (Noszczyk-Nowak, 2012a; Harris et al., 2017). An interesting observation is that studies of dogs with DCM and dogs with subaortic stenosis (SAS) reported mean TO value <0, while dogs with DCM plus CHF present TO < 0 as well (Noszczyk-Nowak, 2012a, 2012b; Harris et al., 2017). In dogs with DCM in a few patients, TO was positive during the night, but on average, the TO value for all analyzed VPCs was negative (Noszczyk-Nowak, 2012a). This is in contrast to patients C and D in this study that had mean TO value over 0. In human medicine, HRT parameters are considered normal when TO <0% (Bauer et al., 2008). This comes in accordance with the TO value for stage D dogs, this was the only group with TO mean ± SD over zero. This worse response of TO by MMVD patients might be due to the slow progress of MMVD when compared to DCM leading to more intense autonomic imbalance. Chronic activation of compensatory mechanism results in a state of unbalanced compensatory response leading to myocardial depletion of NE, abnormal baroreflex, and reduced expression of B-adrenergic cell surface receptors and once signs of CHF develop, the condition is generally progressive and terminal (Tilley et al., 2008). Dogs with MMVD have a period of around 766 days after diagnosis before signs of cardiac disease or death (Boswood et al., 2016). In other hand, some patients with DCM may have less than 30 days of life after diagnosis and can even present sudden death (Noszczyk-Nowak, 2012a). Doberman pinschers with DCM usually develop clinical signs around 50 days after diagnosis, while the average survival time in cocker spaniels is 537 days. It is known that early treatment increases survival time of MMVD affected dogs (Luis-Fuentes et al., 2002; Martin et al., 2010). For the second HRT parameter, the slope, dogs’ stages B1 and B2 had TS over 25 ms/RR reflecting the lower autonomic impact of MMVD. In other hand dogs with MMVD stage C and D showed results lower than 25 ms/RR, that is under the normality threshold suggested in human medicine. For humans the TS is considered normal when >25 ms/RR (Bauer et al., 2008). Other studies of dogs with DCM and SAS reported a TS under 25 ms/RR, and even the control groups of these studies had mean TS values under this value (Noszczyk-Nowak, 2012a, 2012b; Harris et al., 2017). This results raises questions such as if there are differences in HRT of small dogs compared to HRT in bigger dogs and if different conditions like environmental or age related could result in significant differences in HRT. It could also be due to an autonomic imbalance in the control animals of these studies although one of the studies tested n-terminal pro-brain natriuretic peptide (NT-pro-BNP) in the control population and the dogs studied were not on medical therapy (Noszczyk-Nowak, 2012b). This shows the necessity of HRT studies for better comprehension of this autonomic indicator. When HRT is compared to common echocardiographic measurements, TO had weak positive correlations, while TS had three moderate negative correlations and two negative correlations and a single positive weak correlation. Both TO and TS had correlation with atrial size, while TO had weak correlation the TS had moderate correlation with LA/Ao ratio. The HRT showed correlation to left ventricle filling pressures, suggesting volume overload may influence HRT. The TS had stronger correlations than TO with echocardiographic parameters, this suggests that TS is more sensitive, as it had moderate negative correlation to E/IVRT, maximal E wave velocity and the left atrium/aorta ratio. This comes in concordance with other studies affirming that MMVD patients have increase in left atrial size as the disease advances along with mitral E wave maximal velocity and E/A ratio increased in congestive stages (Kim et al., 2018). This suggestion is supported by E/IVRT correlation which suggests that the neurohumoral derangement leads to the activation of the sympathetic nervous system and the consequent hypervolemic state has a negative impact on the relation between left ventricular filling time and filling pressure. There was also a weak positive relation between TS and the IVRT. The TS weak correlation with the E/A ratio and the E/E´ ratio suggest that diastolic function is important in the baroreflex, notably there would be some influence of the myocardial E´ diastolic velocity when we talk about E/E´. The AUC for HRT to differentiate between asymptomatic dogs and symptomatic dogs and to differentiate dogs with non-remodeled hearts from dogs with remodeled hearts was within 0.79 and 0.89 range. It was also noted that the AUC for TO and TS were more expressive to differentiate between asymptomatic patients from symptomatic patients, than to differentiate dogs with non-remodeled hearts from dogs with remodeled hearts. This diminished HRT shows that late stages characterized by CHF have accentuated autonomic imbalance. The general AUC for HRT parameters was between 0.79 and 0.89, that should be considered a very good result (Carter et al., 2016). The HRT holds similar or even greater AUC than other diagnostic parameters in echocardiography or seric NT-pro-BNP. Accordingly, Schober et al. (2010) commonly used echocardiography parameters as E:A ratio, maximal transmitral E wave velocity and IVRT have an AUC of 0.86–0.89 while the greatest AUC among echocardiography parameters was E:IVRT. The same study found that seric NT-pro-BNP has an AUC of 0.83. This study was not designed to test the influence of coupling interval or compensatory pause on HRT parameters. The role of these two variables is controversial, with some studies showing that they may exert weak influence, while HR may have greater effect on HRT results (Tilley et al., 2002; Stöckigt et al., 2014). There are also limitations in this study, since no normality value was collected for dogs in stage A, it was not possible to compare the results among all the MMVD stages. Retrospective collection of data presents challenges in interpretation as it is possible to ensure consistent quality of data because the examinations were performed by a number of different operators. Another challenge in this study was data collection, because of the limitation that some patients had less than five VPCs to be present for the HRT calculation, which is the standard technique described for human medicine, while excessive numbers of VPCs can disturb the sinus rhythm. This difficulty was described in another article involving HRT in dogs (Harris et al., 2017), but a third study did not find this as a limitation (Noszczyk-Nowak et al., 2012b). In our cohort stage, B1 dogs had very few VPCs in the electrocardiogram and the holter exam was not always available due to time and cost demands. It was hard to acquire sufficient patients in Class D as well, as suitable patients declined to participate due to the continuous 24 hour exam requirement. In this study, we did not evaluate if there are differences in HRT of brachycephalic and non-brachycephalic dogs, but it was shown that brachycephalic dogs can present greater R-R intervals and have greater VVTI than non-dolichocephalic dogs (Doxey and Boswood, 2004). Also medication effect on HRT was not analyzed; however, studies suggest that medication may affect autonomic balance. It shown that 90 days of sildenafil may improve parasympathetic activity in dogs with asymptomatic MMVD, although in a 180 days comparison there was no difference found between control, sildenafil or enalapril groups (Pirintr et al., 2017). Also, there is evidence short term therapy in dogs with enalapril have significantly reduced sympathetic activity (Chompoosan et al., 2014). In humans with infarct after taking beta-blockers for 12 months abnormal fractal HR behavior and the slope of HRT still predicted cardiac mortality (Jokinen et al., 2003). The physical activities and daily routine were not evaluated, but they could influence the dogs autonomic balance by increasing parasympathetic activity (Valandro et al., 2017). Currently, dogs affected by MMVD are classified based on: Stage A predisposition; Stage B1 structural remodeling not present or not severe enough to meet the trial criteria; Stage B2—Radiographic, echocardiographic evidence of atrial or ventricular enlargement, in this stage is recommended to start the treatment; Stage C—Denotes dogs with current or past clinical signs of HF: D—Refers to end-stage refractory patients (Keene et al., 2019). Before the onset of CHF, the heart size can be assessed by vertebral heart score (VHS) and echocardiography and in vital signs we can see changes in HR and RR. The HR seems to change earlier than RR (Boswood et al., 2020). But clinical signs may be misinterpreted by owners are highly subjective and some may be seen in association with respiratory disease (Atkins et al., 2009). Furthermore, other studies are required for better comprehension of HRT in dogs with MMVD without other diseases according to their HRT results, HRT could be used to further stratify B1 stage or could be used to monitor dogs that are in the worst conditions and at higher risk of dying. To the authors knowledge, this is the first study of HRT in dogs with MMVD, and further studies are required to investigate HRT behavior in different scenarios. In humans, it is known that HRV can be affected by a vast amount of conditions like environmental, physiological, lifestyle, and biologic factors such as genetics and metabolism. Pathologic conditions as well play a role in the autonomic balance as it can be seen in HRV of humans with sepsis, respiratory, renal or heart disease (Samito et al., 2016). Humans with cardiac autonomic neuropathy (CAN) due to type 2 diabetes mellitus have significantly diminished TS when compared to individuals without CAN (Balcıoğlu et al., 2007). In the future maybe changes in TO and TS could be used for further assessment of autonomic balance of dogs with MMVD. The conclusions are that HRT is a feasible tool for assessing the autonomic balance in dogs, the HRT changes as the MMVD and CHF progress, the HRT overall accuracy is very good and the HRT parameters have low to moderate correlation with some commonly used echocardiographic parameters. Conflict of interestThe authors declare that there is no conflict of interest. AcknowledgmentsThe authors are grateful for the financial support provided by Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES) e Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). ReferencesAtkins, C., Bonagura, J., Ettinger, S., Fox, P., Gordon, S., Haggstrom, J. and Stepien, R. 2009. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J. Vet. Intern. Med. 23(6), 1142–1150. Balcıoğlu, S., Arslan, U., Türkoǧlu, S., Özdemir, M. and Çengel, A. 2007. Heart rate variability and heart rate turbulence in patients with type 2 diabetes mellitus with versus without cardiac autonomic neuropathy. Am. J. Cardiol. 100(5), 890–893. Bauer, A., Malik, M., Schmidt, G., Barthel, P., Bonnemeier, H., Cygankiewicz, I. and Zareba, W. 2008. Heart rate turbulence: standards of measurement, physiological interpretation, and clinical use. International Society for Holter and Noninvasive Electrophysiology Consensus. J. Am. Coll. Cardiol. 52(17), 1353–1365. Behrend, E.N., Kooistra, H.S., Nelson, R., Reusch, C.E. and Scott-Moncrieff, J.C. 2013. Diagnosis of spontaneous canine hyperadrenocorticism: 2012 ACVIM consensus statement (small animal). J. Vet. Int. Med. 27(6), 1292–1304. Boswood, A., Häggström, J., Gordon, S.G., Wess, G., Stepien, R.L., Oyama, M.A. and Watson, P. 2016. Effect of pimobendan in dogs with preclinical myxomatous mitral valve disease and cardiomegaly: the epic study—a randomized clinical trial. J. Vet. Med. 30(6), 1765–1779. Boswood, A., Gordon, S.G., Häggström, J., Vanselow, M., Wess, G., Stepien, R.L., Oyama, M.A., Keene, B.W., Bonagura, J., MacDonald, K.A., Patteson, M., Smith, S., Fox, P.R., Sanderson, K., Woolley, R., Szatmári, V., Menaut, P., Church, W.M., O’Sullivan, M.L. and Watson, P. 2020. Temporal changes in clinical and radiographic variables in dogs with preclinical myxomatous mitral valve disease: the epic study. J. Vet. Int. Med. 34(3), 1108–1118. Borgarelli, M., Savarino, P., Crosara, S., Santilli, R.A., Chiavegato, D., Poggi, M. and Tarducci, A. 2008. Survival characteristics and prognostic variables of dogs with mitral regurgitation attributable to myxomatous valve disease. J. Vet. Med. 22, 120–128. Brüler, B.C., Vieira, T.C., Wolf, M., Lucina, S.B., Montiani-Ferreira, F. and Sousa, M.G. 2018. Using the oculocardiac reflex to characterize autonomic imbalance in a naturally occurring canine model of valvular insufficiency. Comp. Med. 68(2), 156–162. Carter, J.V., Pan, J., Rai, S.N. and Galandiuk, S. 2016. ROC-ing along: evaluation and interpretation of receiver operating characteristic curves. Surgery 159(6), 1638–1645. Chompoosan, C., Buranakarl, C., Chaiyabutr, N. and Chansaisakorn, W. 2014. Decreased sympathetic tone after short-term treatment with enalapril in dogs with mild chronic mitral valve disease. Res. Vet. Sci. 96(2), 347–354. Davies, L.C., Francis, D.P., Ponikowski, P., Piepoli, M.F. and Coats, A.J.S. 2001. Relation of heart rate and blood pressure turbulence following premature ventricular complexes to baroreflex sensitivity in chronic congestive heart failure. Am. J. Cardiol. 87(6), 737–742. Doxey, S. and Boswood, A. 2004. Differences between breeds of dog in a measure of heart rate variability. Vet. Rec. 154(23), 713–717. Eckberg, D.L., Drabinsky, M. and Braunwald, E. 1971. Defective cardiac parasympathetic control in patients with heart disease. N. Engl. J. Med. 285, 877–883. Fox, P.R. 2012. Pathology of myxomatous mitral valve disease in the dog. J. Vet. Cardiol. 14, 103–126. Gunduz, H., Arinc, H., Kayardi, M., Akdemir, R., Ozyildirim, S. and Uyan, C. 2006. Heart rate turbulence and heart rate variability in patients with mitral valve prolapse. Europace 8(7), 515–520. Harris, J.D., Little, C.J.L., Dennis, J.M. and Patteson, M.W. 2017. Heart rate turbulence after ventricular premature beats in healthy Doberman pinschers and those with dilated cardiomyopathy. J. Vet. Cardiol. 19(5), 421–443. IRIS (International Renal Interest Society). 2019. Iris staging of CKD (modified 2019). Available via http://www.iris-kidney.com/guidelines/ (Accessed 27 June 2021). Jokinen, V., Tapanainen, J.M., Seppänen, T. and Huikuri, H.V. 2003. Temporal changes and prognostic significance of measures of heart rate dynamics after acute myocardial infarction in the beta-blocking era. Am. J. Cardiol. 92(8), 907–912. Keene, B.W., Atkins, C.E., Bonagura, J.D., Fox, P.R., Häggström, J., Fuentes, V.L., Oyama, M.A, Rush, J.E., Stepien, R. and Uechi, M. 2019. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J. Vet. Int. Med. 33(3), 1127–1140. Kim, Y.H., Choi, G.J. and Park, C. 2018. Rate of left ventricular pressure change by Doppler echocardiography in dogs with chronic mitral valve disease at different stages of congestive heart failure. Vet. Radiol. Ultrasound 59(6), 758–766. Lin, L.Y., Lai, L.P., Lin, J.L., Du, C.C., Shau, W.Y., Chan, H.L., Tseng, Y.Z. and Huang, S.K.S. 2002. Tight mechanism correlation between heart rate turbulence and baroreflex sensitivity: sequential autonomic blockade analysis. J. Cardiovasc. Electrophysiol. 13(5), 427–431. Luis-Fuentes, V., Corcoran, B., French, A., Schober, E.K., Kleemann, R. and Justus, C. 2002. A double-blind, randomized, placebo-controlled study of Pimobendan in dogs with dilated cardiomyopathy. J. Vet. Med. 16, 255–261. Lymperopoulos, A., Rengo, G. and Koch, W.J. 2013. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ. Res. 113, 739–753. Marine, J.E., Watanabe, M.A., Smith, T.W. and Monahan, K.M. 2002. Effect of atropine on heart rate turbulence. Am. J. Cardiol. 89, 767–769. Martin, M.W.S, Stafford, J.M., Strehlau, G. and King, J.N. 2010. Canine dilated cardiomyopathy: a retrospective study of prognostic findings in 367 clinical cases. J. Small Anim. Pract. 51(8), 428–436. Noszczyk-Nowak, A. 2012a. Heart rate turbulence in healthy dogs and dogs with dilated cardiomyopathy. Pol. J. Vet. Sci. 15(3), 469–475. Noszczyk-Nowak, A. 2012b. Heart rate turbulence in mild-to-moderate aortic stenosis in boxers. Pol. J. Vet. Sci. 15(3), 477–481. Öztürk, C., Schueler, R., Weber, M., Welz, A., Werner, N., Nickenig, G. and Hammerstingl, C. 2016. Sympathetic activity in patients with secondary symptomatic mitral regurgitation or end-stage systolic heart failure. JACC Cardiovasc. Interv. 9(19), 2050–2057. Pirintr, P., Saengklub, N., Limprasutr, V., Sawankoon, S. and Kijtawornrat, A. 2017 Sildenafil improves heart rate variability in dogs with asymptomatic myxomatous mitral valve degeneration. J. Vet. Med. Sci. 79(9), 1480–1488. Schmidt, G., Malik, M., Barthel, P., Schneider, R., Ulm, K., Rolnitzky, L. and Schömig, A. 1999. Heart-rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction heart rate turbulence and heart rate variability in patients with mitral valve prolapse. Lancet 353(9162), 1390–1396. Stöckigt, F., Pöhlmann, S., Nickenig, G., Schwab, J.O. and Schrickel, J.W. 2014. Induced and spontaneous heart rate turbulence in mice: influence of coupling interval. Europace 16(7), 1092–1098. Tilley, L.P. Smith, F.W.K., Oyama, M.A. and Sleeper, M. 2008. Manual of canine and feline cardiology. Elsevier Health Sciences. TFESCNASPE (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology). 1996. Heart rate variability: standards of measurements, physiological interpretation, and clinical use. Eur. Heart J. 17(3), 354–381. Valandro, M.A., Pascon, J.P.E., Pereira, D.T.P. and Mistieri, M.L.A. 2017. Exercise training of dogs with myxomatous valve disease. Arq. Bras. Med. Vet. Zootec. 69(2), 325–332. Wichterle, D., Melenovsky, V. and Malik, M. 2002. Mechanisms involved in heart rate turbulence. Card. Electrophysiol. Rev. 6(3), 262–266. | ||
| How to Cite this Article |
| Pubmed Style Santos JPD, Costa BND, Lucina SB, Olaguivel KC, Tulesky GLR, Sousa MG. Assessment of heart rate turbulence in dogs with myxomatous mitral valve disease. Open Vet. J.. 2021; 11(4): 635-644. doi:10.5455/OVJ.2021.v11.i4.13 Web Style Santos JPD, Costa BND, Lucina SB, Olaguivel KC, Tulesky GLR, Sousa MG. Assessment of heart rate turbulence in dogs with myxomatous mitral valve disease. https://www.openveterinaryjournal.com/?mno=33140 [Access: January 25, 2026]. doi:10.5455/OVJ.2021.v11.i4.13 AMA (American Medical Association) Style Santos JPD, Costa BND, Lucina SB, Olaguivel KC, Tulesky GLR, Sousa MG. Assessment of heart rate turbulence in dogs with myxomatous mitral valve disease. Open Vet. J.. 2021; 11(4): 635-644. doi:10.5455/OVJ.2021.v11.i4.13 Vancouver/ICMJE Style Santos JPD, Costa BND, Lucina SB, Olaguivel KC, Tulesky GLR, Sousa MG. Assessment of heart rate turbulence in dogs with myxomatous mitral valve disease. Open Vet. J.. (2021), [cited January 25, 2026]; 11(4): 635-644. doi:10.5455/OVJ.2021.v11.i4.13 Harvard Style Santos, J. P. D., Costa, . B. N. D., Lucina, . S. B., Olaguivel, . K. C., Tulesky, . G. L. R. & Sousa, . M. G. (2021) Assessment of heart rate turbulence in dogs with myxomatous mitral valve disease. Open Vet. J., 11 (4), 635-644. doi:10.5455/OVJ.2021.v11.i4.13 Turabian Style Santos, Julio Pereira Dos, Bruna Natali Da Costa, Stephany Bulba Lucina, Karla Calderon Olaguivel, Giovana L. R. Tulesky, and Marlos Goncalves Sousa. 2021. Assessment of heart rate turbulence in dogs with myxomatous mitral valve disease. Open Veterinary Journal, 11 (4), 635-644. doi:10.5455/OVJ.2021.v11.i4.13 Chicago Style Santos, Julio Pereira Dos, Bruna Natali Da Costa, Stephany Bulba Lucina, Karla Calderon Olaguivel, Giovana L. R. Tulesky, and Marlos Goncalves Sousa. "Assessment of heart rate turbulence in dogs with myxomatous mitral valve disease." Open Veterinary Journal 11 (2021), 635-644. doi:10.5455/OVJ.2021.v11.i4.13 MLA (The Modern Language Association) Style Santos, Julio Pereira Dos, Bruna Natali Da Costa, Stephany Bulba Lucina, Karla Calderon Olaguivel, Giovana L. R. Tulesky, and Marlos Goncalves Sousa. "Assessment of heart rate turbulence in dogs with myxomatous mitral valve disease." Open Veterinary Journal 11.4 (2021), 635-644. Print. doi:10.5455/OVJ.2021.v11.i4.13 APA (American Psychological Association) Style Santos, J. P. D., Costa, . B. N. D., Lucina, . S. B., Olaguivel, . K. C., Tulesky, . G. L. R. & Sousa, . M. G. (2021) Assessment of heart rate turbulence in dogs with myxomatous mitral valve disease. Open Veterinary Journal, 11 (4), 635-644. doi:10.5455/OVJ.2021.v11.i4.13 |