| Original Article | ||
Open Vet. J.. 2021; 11(4): 544-554 Open Veterinary Journal, (2021), Vol. 11(4): 544–554 Original Research Effects of hyperbaric oxygen therapy on wound healing in veterinary medicine: A pilot studyDébora Gouveia1†, Sara Bimbarra1,2†, Carla Carvalho1, Ana Cardoso1, Óscar Gamboa3, Rute Teixeira2, António Ferreira3 and Ângela Martins1,2†*1Arrábida Veterinary Hospital—Animal Rehabilitation Center, Azeitão, Portugal 2Faculty of Veterinary Medicine, Lusófona University, Lisboa, Portugal 3Faculty of Veterinary Medicine, University of Lisbon, Lisboa, Portugal †These authors contributed equally to this work *Corresponding Author: Ângela Martins. Faculty of Veterinary Medicine, Lusófona University, Lisboa, Portugal. Email: vetarrabida.lda [at] gmail.com Submitted: 03/06/2021 Accepted: 17/09/2021 Published: 10/10/2021 © 2021 Open Veterinary Journal
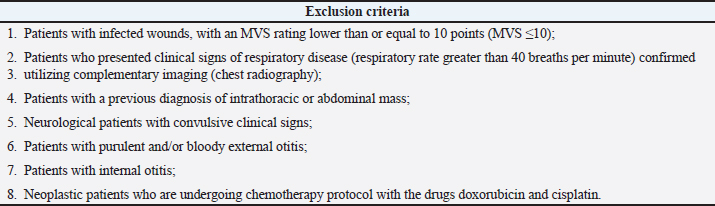
AbstractBackground: In veterinary medicine, wounds have a high incidence in clinical practice. A technique that can accelerate healing has been extensively studied, and the treatment with hyperbaric oxygen therapy (HBOT) is currently recognized as one of the best adjuvant treatments in this matter. Aim: The main objective of this pilot clinical study was to assess the therapeutic effect of HBOT in severe wounds classified according to the Modified Vancouver Scale (MVS) between 10 and 15 points or greater than 15 points (MVS > 10 and ≤ 15; MVS > 15). Methods: A study population of 41 patients was divided into the dog group and the cat group and were treated at Lisbon Animal Rehabilitation and Regeneration Center, with 100% oxygen and 2.4 atmospheres absolute for 90 minutes. The patients’ wounds were assessed using the MVS at the time of admission, in the first 24 hours, 48 hours, 72 hours after HBOT, and at the time of medical release. This study also sought to assess if HBOT is a safe therapy in small animal clinical practices by monitoring the major side effects (SEM) and minor side effects (SEm) observed throughout each session. Results: The results obtained showed that HBOT allowed a decrease in the MVS classification. Conclusion: The results suggested that HBOT may be an interesting complementary therapy to be prescribed in wounds that present difficulty in healing. Furthermore, it was considered a safe therapy since in 289 sessions of HBOT, no SEM was observed, and as for SEm, the highest incidence was the act of swallowing. However, more studies should be carried out with HBOT in small animal clinical practices to confirm these results. Keywords: Cat, Dog, Hyperbaric oxygen therapy, Modified Vancouver Scale (MVS), Wound healing. IntroductionThe healing process occurs to restore the anatomical and functional integrity of the tissue. It is considered a systemic process in which the main cause of non-healing is the interaction between tissue hypoperfusion and infection, with a risk factor caused by hypoxia and low oxygen content (Ferreira et al., 2008; Bitterman, 2009). In veterinary medicine, wounds are important due to their high incidence, regardless of the species, breed, and size. The possibility of accelerating the healing process and treating difficult wounds has been studied, and the treatment with hyperbaric oxygen therapy (HBOT) is currently recognized as one of the best adjuvant treatments in this field (Roberto and Barbosa, 2019). The HBOT consists of the administration by inhalation of a high dose of oxygen (100%) inside a hyperbaric chamber with a pressure that can range from 1.4 to 3 atmospheres absolute (ATA) (Memar et al., 2019). The HBOT can be used as a treatment and, therefore, prescribed with a specific dose with also potential side effects and contraindications. Hyperbaric treatment has a minimum effective concentration (1.4 ATA) and a maximum effective concentration (3 ATA), from which oxygen plays a toxic role (Sheffield and Smith, 2002; Jokinen-Gordon et al., 2017). The HBOT at 3 ATA promotes a plasma oxygen concentration of approximately 60 ml/l, which may help ischemic tissue and poorly oxygenated wounds (Jones and Cooper, 2021). High oxygen concentrations favor the complete saturation of hemoglobin molecules in the bloodstream (Hengel et al., 2013). The increased oxygen dissolved in the plasma stimulates angiogenesis (Pavletic, 2018), contributes to several cellular processes involved during the healing process, and blocks microorganism development (Gesell, 2008; Shah, 2010). The normal oxygen solubility in plasma is 0.24 ml of O2/dl. This value is variable under hyperbaric conditions due to the effect of pressure. When 2.0 ATA is applied, it is possible to increase the amount of oxygen dissolved in the plasma to 4.4 ml/dl; with 2.5 ATA, it is possible to reach values of 5.6 ml of O2/dl (Çimşi et al., 2009), and with 3.0 ATA—the maximum pressure considered safe for administration—a value of 6.8 ml of O2/dl can be reached (Choudhury, 2018). Table 1. Exclusion criteria for the study population selection.
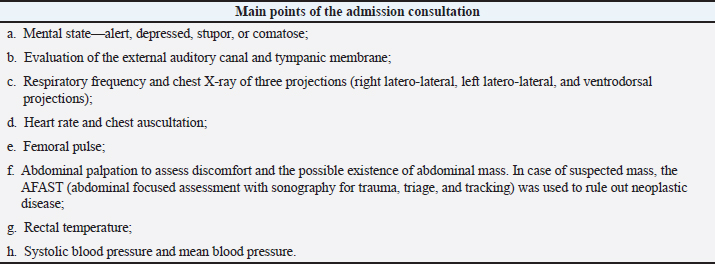
The HBOT has a bactericidal and bacteriostatic role (Knighton et al., 1984; Jones and Cooper, 2021), further enhancing leukocyte activity, especially with regard to oxidative death mechanisms (Zamboni et al., 2003). Under hyperbaric conditions, it has also been documented that there is a potentiating effect of antibiotics (Çimşi et al., 2009). The HBOT promotes the production of vascular endothelial growth factor (VEGF), stimulating healing by producing a larger capillary network. Neovascularization is essential to replace new tissue, promoting the migration of fibroblasts and epithelial cells. In addition, it activates fibroblasts and macrophages, reduces edema, and promotes collagen synthesis and angiogenesis (Abdullah et al., 2006). Angiogenesis under hyperbaric conditions has already been documented in several skin lesions such as burns (Bilic et al., 2005), after plastic surgery (Zhang et al., 2007), wounds (Arikan et al., 2005; Klemetti et al., 2005), and bone healing (Muhonen et al., 2004). For this pilot clinical study, it was hypothesized that wounds that are difficult to heal with a score higher than 10 points according to the Modified Vancouver Scale (MVS) might benefit from the action of HBOT so that throughout its temporal evolution, they present a decrease in the MVS classification being indicative of improvement with regard to the severity of the lesion. Our study had three main objectives: the first was to adapt the Vancouver Scar Assessment Scale (VSS) from human medicine to the veterinary clinical practice to create a tool for monitoring healing in small animals; the second, to verify if HBOT may be a safe therapy in the clinical practice of small animals; the last and third aim was to assess if HBOT may have a possible therapeutic action in severe wounds classified according to the MVS between 10 and 15 points (MVS > 10 and ≤ 15) and greater than 15 points (MVS > 15). Patients were evaluated at admission to the study (MVST0) and immediately underwent HBOT. The following evaluations were carried out in the first 24 hours (MVST1), 48 hours (MVST2), and 72 hours (MVST3) after therapy, and at the time of medical release (MVST4). The moment of medical release was defined as the moment corresponding to the maximum possible healing, obtaining MVS values from good to a very good prognosis. Materials and MethodsThis pilot clinical study was conducted at Arrábida Veterinary Hospital (HVA) and Lisbon Animal Rehabilitation and Regeneration Center (CR2AL) between March 2, 2020, and February 2, 2021. The present clinical study addresses dogs and cats’ injuries, regardless of age, weight, sex, breed, and etiology of the injury. Although the lesions’ etiologies were variable and independent of the study population, all patients showed clinical signs of severe wounds with a reserved/bad prognosis according to the MVS classification. Study populationThe clinical study consisted of 41 patients represented by 25 dogs—with an average age of 8.1 years, and an average weight of 22.6 kg, in which 11 were male and 14 were female—and by 16 cats—with an average age of 4.4 years and an average weight of 3.8 kg, in which 8 were male and 8 were female. For the study, dogs and cats with the following criteria were selected: patients with infected wounds, with or without bone involvement, with an MVS classification of reserved prognosis (MVS > 10 and ≤ 15) to bad (MVS > 15) and patients with infected wounds, regardless of the time elapsed from the injury occurred to the admission to the study. Regarding exclusion criteria, they are described in Table 1. Admission consultationThe initial study population consisted of 75 patients (n=75). After the admission consultation and considering the selection and exclusion criteria, 41 patients (n=41) were selected for the study population, including 25 dogs (n=25) and 16 cats (n=16). Thus, two study groups were created: the dog group (Gd) and the cat group (Gc). The patients admitted to the study were hospitalized at HVA and subsequently were submitted to a physical examination and assessment of vital parameters. The main points of the admission consultation are described in Table 2. Table 2. Main points of the admission consultation.
Wound assessment and protective dressingTo evaluate the infected wound, trichotomy of the injured region was carried out and later cleaned with isotonic serum associated with 2% chlorhexidine. At the end of this process, a bandage was applied to protect the wound. Commercial honey was then applied, coated with sterile compresses and gauze bandages, and fixed with perforated adhesive. The bandage was used not to tighten the injured area but to prevent ischemia, edema, or cell death. Internal medicine protocolAll Gd and Gc patients were submitted to biochemical analyses (albumin, total protein, urea, creatinine, alanine aminotransferase, and globulins) with associated blood count and coagulation times (activated partial thromboplastin time and prothrombin time).
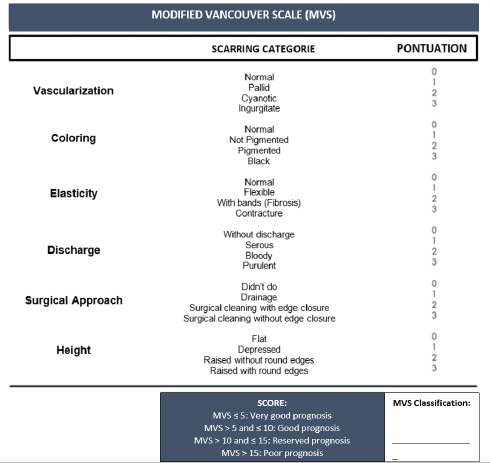
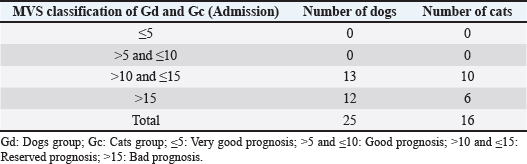
Modified Vancouver ScaleIn this pilot study, it was necessary to adapt to a scale to monitor wound healing evolution. Thus, the association between human medicine scales, such as the VSS (Fearmonti et al., 2010) and the Southampton Scale (Gottrup et al., 2005), resulted in a scale for veterinary medicine purposes, the MVS. The MVS allows wounds’ assessment and their prognosis as a scoring scale. It includes six parameters: vascularization, coloring, elasticity, discharge, surgical approach, and height. For each parameter, four scarring categories were assigned with a score between 0 and 3 (Fig. 1). The sum of the points attributed to the different parameters can vary between 0 and 18 points in total, with a classification below or equal to 5 points (MVS ≤ 5) corresponding to a very good prognosis; a rating greater than 5 points and less than or equal to 10 points (MVS > 5 and ≤ 10) corresponds to a good prognosis; a rating greater than 10 points and less than or equal to 15 points ( MVS > 10 and ≤ 15) corresponds to a reserved prognosis; and a score higher than 15 points (MVS > 15) corresponds to a poor prognosis. Table 3 shows the MVS classification of both Gd and Gc groups, respectively, at the time of admission to the study. The HBOT protocolThe day after admission, patients were transported to CR2AL and started the HBOT protocol after consent by the tutor by completing the consent form, which was a mandatory requirement. Given the severity of the injuries, it was suggested that in an initial approach, the patient should be given between 5 and 10 sessions of HBOT, depending on the type of wound, surrounding anatomical region, and tutor financial possibilities. It should be noted that all consultations were carried out by the same veterinarian, accredited by Hyperbaric Veterinary Institute (HVI®) and certified by the International ATMO®, as well as an instructor of the Certified Canine Rehabilitation Practitioner CCRP. The HBOT protocol applied to the study population had to consider the pre-therapeutic care and the HBOT itself. Regarding pre-therapeutic care, before the patients step in to the hyperbaric chamber, they were sprayed and moistened with water, and all the pectorals, collars, and metallic accessories were removed to reduce the risk of ignition associated with HBOT. In case wound protection bandages and intravenous catheters, they were carefully protected with 100% cotton bands. Patients in groups Gd and Gc did not need any pharmacological administration such as sedatives and/or anesthetics before starting HBOT.
Fig. 1. Modified Vancouver Scale (MVS). Table 3. MVS classification of both groups Gd and Gc at the moment of admission.
According to the protocol, when connecting the hyperbaric chamber, it was mandatory to test for its “grounding,” with values between 0 and 1 ohm (Ω), as the standard values to ensure safety. The therapy with HBOT itself was carried out in a single-position hyperbaric chamber type C, consisting of two bilateral eye windows and a television monitor that allows live transmission through two digital cameras presented inside the hyperbaric chamber, Hyperbaric Veterinary Medicine (HVM®) (Fig. 2). The hyperbaric chamber used was provided by a system made by an oxygen bottle circuit with 200 bars each, having two groups of three bottles. This system was certified by Linde®. Treatments followed the guidelines existing in CR2AL and were carried out every 24 hours with a duration between 60 and 90 minutes and 1 treatment per day. The number of sessions per patient was variable. The therapeutic range considered in the present study was 2.4 ATA, and patients were subjected, in all treatments, to compression and decompression protocols. The reduction was first carried out for about 15–20 minutes during the therapeutic process, followed by the treatment itself. In the final phase, the decompression was carried out, which lasted 20–25 minutes. This protocol was adjusted according to the daily clinical exam.
Fig. 2. Hyperbaric chamber model (Photograph kindly provided by CR2AL). Side effects monitoringPatients were monitored during each HBOT session for side effects, and SEM and SEm were recorded in a patient monitoring table. The side effects were divided into minor side effects (SEm), such as vocalize, yawn, swallow, and ear and head scratch, and major side effects (SEM), such as barotrauma, convulsive state, syncope, and death. Statistical analysisThe data collected for the present study were recorded in Microsoft Office Excel 2016® spreadsheets (Microsoft®, USA) to characterize the sample by analyzing the frequency of the various categorical variables. For the interferential statistical analysis, the IBM SPSS Statistics 2020® program was used in which chi-square tests were carried out for crossing and evaluating the relationships between several categorical variables. Ethical approvalThis study was approved by the ethics committee Comissão de Ética e Bem Estar Animal of the Faculty of Veterinary Medicine, Lusofona University. ResultsDescriptive analysisThe population sample consisted of 41 patients, of which 61% (25/41) were dogs and 39% (16/41) were cats. With regard to sex, the study included 19 male (46%) and 22 female (54%). With regard to weight, the average sample was 15.29 kg (minimum weight of 3 kg; maximum weight of 50 kg), with a median of 14. As for age, the average was 6.6 years (minimum age 1 year; maximum age 16 years) with a median of 6. The population sample was evaluated for the number of wounds infected with bone involvement with a prevalence of 44% (18/41). All of these dogs did, as part of their antibiotic therapy admission protocol, the clindamycin, and of them, 16 patients experienced culture and antibiogram, changing antibiotics after the result. The other 56% (23/41), which did not have bone involvement, experienced the antibiotic therapy protocol with ampicillin, enrofloxacin, and metronidazole. The study population was also evaluated for hospitalization time, with 24% (10/41) of the patients hospitalized for less than 7 days and 76% (31/41) of the patients hospitalized for more than 7 days. With regard to culture and cytology, for antibiogram, only 39% (16/41) underwent this procedure. During the hospitalization process, 54% (22/41) required surgical cleaning to remove the necrotic tissue gradually. HBOT was considered a safe therapyDuring the HBOT sessions, SEM was not observed in a total of 289 sessions carried out. As for SEm, the swallowing effect was present in 24% (10/41) of the study population, followed by vocalization and yawning with a prevalence of less than 20% (7/41). Regarding the 289 sessions of HBOT, 161 sessions were carried out in dogs, while in cats, only 128 sessions were carried out. The average number of sessions per patient was six, with a median of 5. The number of sessions and the median were the same for dogs and cats, but only a small change in the median value was six sessions in cats.
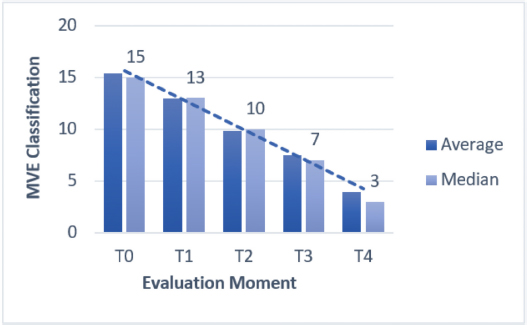
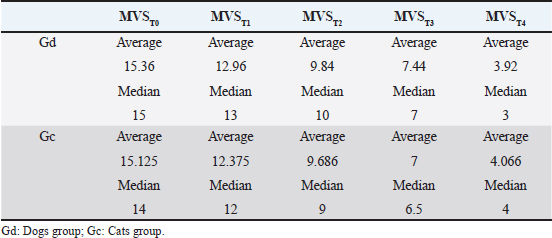
Fig. 3. Average and median MVS classification in MVST0, MVST1, MVST2, MVST3, and MVST4. HBOT allowed a decrease in the MVS classificationEvaluation and classification of the wounds were carried out in five moments (MVST0, MVST1, MVST2, MVST3, and MVST4). The evolutions throughout the moments were evaluated using the MVS, which can be seen in Figure 3. HBOT promoted wound healingIn the MVST4 classification, it was found that 73% (30/41) of the patients obtained an MVS less or equal to 5 points (MVS ≤ 5), indicative of a very good prognosis, and 27% (11/41) demonstrated MVS value greater than 5 and less or equal to 10 points (MVS > 5 and ≤ 10), indicative of good prognosis (Fig. 4), suggesting that at the end of the study 100% of the patients had a good prognosis indicative of clinical success. Throughout the study, a regression rate equivalent to 80% (33/41) was recorded in relation to MVST0 and MVST4 (Fig. 5). HBOT promoted the acceleration of the wound healing process. regardless of speciesThe present study demonstrated the correlation between the average and median of the MVST0, MVST1, MVST2, MVST3, and MVST4 classifications between the Gd group and the Gc group, as shown in Table 4. Regarding the interferential statistical analysis, the correlation between MVST0 and hospitalization time had no statistical significance (X2 (1, N=41)=3.1, p= 0.08), although there was a strong relationship of significance (X2 (1, N=41)=5.3, p=0.02) between species and hospitalization time. The existence of concomitant diseases did not obtain statistical significance when crossed with hospitalization time (X2 (1, N=41)=0.6, p=0.41) and the age category (X2 (2, N=41)=0.90, p=0.64). The faster the patients were admitted to the hospital, the better was their MVS rating at medical releaseThe time from the injury to admission to the study, when correlated with the MVST4, showed a trend of significance (X2 (1, N=41)=3.6, p=0.05), wherein 76% (31/41) of the patients admitted to the hospital shortly after the injury obtained a very good evaluation at the medical release in regard to the MVS. Worse MVS classification at 24 hours (MVST1) promoted longer hospitalization timeThe study used the MVS as a tool to classify patients’ injuries and their evolution. Therefore, there was a strong relationship of significance (X2 (2, N=41)=10.6, p=0.005) between MVST1 classification and hospitalization time. Regarding the 20 patients who had a poor prognosis in MVST1 (MVS > 10 and ≤ 15), 18 of them required a hospital stay longer than 7 days, as well as 8 of the 9 patients showed poor prognosis (MVS > 15). It was also found that 76% (31/41) of the study population required a hospitalization period of more than 7 days until they were released. Gc required fewer HBOT sessions to achieve maximum possible healing when compared to GdIn relation to the Gd and Gc groups, there were statistically significant differences in the Gc group (X2 (1, N=16)=0.01, p=0.01) between the number of HBOT sessions and hospitalization time. Of the eight cats who underwent five or fewer treatments (number of sessions ≤ 5), six were released in less than 7 days of hospitalization and two were released in a period greater than 7 days in the hospital. Of the 8 cats who experienced more than 5 and less than or equal to 10 sessions (number of sessions > 5 and ≤ 10 sessions), only 1 was released within a period of fewer than 7 days and 7 were released after 7 days of hospitalization.
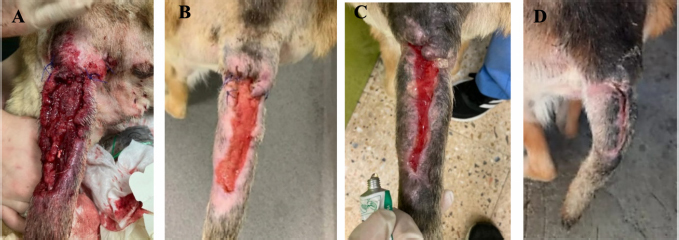
Fig. 4. Evolution of wound at the base of the tail from MVST0 to MVST4. (A) Wound upon admission with MVST0 > 15. (B) Wound after surgical management (cleaning of necrotic tissue and approximation of edges). (C) Wound after 5 HBOT sessions with MVS > 10 and ≤ 15. (D) Wound after 10 HBOT sessions with classification MVST4 > 5 and ≤ 10. Photographs were kindly provided by HVA/CR2AL.
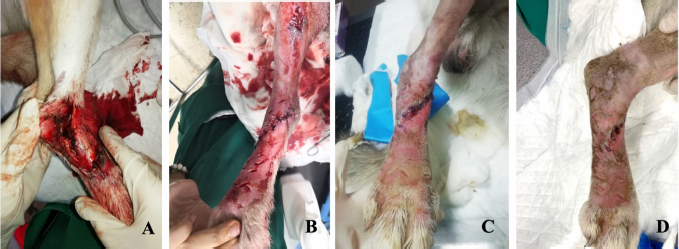
Fig. 5. Wound evolution in the right pelvic limb from MVST0 to MVST4. (A) Wound upon admission with MVST0 > 15. (B) Wound after surgical management (approach of edges). (C) Wound after 5 HBOT sessions with MVS > 10 and ≤ 15. (D) Wound after 10 HBOT sessions with MVST4 ≤ 5. Photographs kindly provided by HVA/ CR2AL. Table 4. Correlation between the average and median MVS classification in MVST0, MVST1, MVST2, MVST3, and MVST4.
DiscussionIn the pilot clinical study, the effect of HBOT on wounds that were difficult to heal was addressed, according to the guidelines of the Undersea Hiperbaric Medicine Society (UHMS®2019) and the Tenth European Consensus Conference on Hiperbaric Medicine. The European Commission on Hyperbaric Medicine indicates different etiologies subjected to this type of therapy, including wound healing. Thus, the chosen oxygen concentration reached values above 1.4 ATA to guarantee therapeutic effects through a high atmospheric pressure that has been standardized between 2.0 and 2.8 ATA (Oyaizu et al., 2018; Kirby et al., 2019). The HBOT protocol defined in this study used 2.4 ATA with therapeutic duration after compression of 90 minutes, according to Bhutani and Vishwanath (2012) and Mazzi (2018). This study showed that 289 sessions of HBOT were carried out, with 161 sessions in Gd and 128 sessions in Gc. This study population of 41 patients was lower than the study by Birnie et al. (2018), although with more HBOT treatments. The study by Birnie et al. (2018) aimed to prove that HBOT allowed safety and tolerance by all dogs and cats, with the average number of treatments per patient being 2.55 sessions. This value was lower than the one found in our study, which was six sessions per patient, also mentioning that in Gd and Gc, the average of six sessions was maintained as the number of average sessions per patient. The mean age in the study was 6.6 years with an average weight of 15.29 kg, with patients weighing a minimum of 3 kg and a maximum weight of 50 kg, since the hyperbaric chamber used (HVM®,) allows treatments to be carried out on dogs up to 80 kg. Bone involvement, with possible osteomyelitis, obtained a prevalence of 44% (18/41), thus indicating an associated healing difficulty since most antibiotics do not penetrate the bone (Handley and Cooper, 2017). Thus, all of these dogs had more time until recovery, and the ones that had repetitive surgical cleaning represented 54% (22/41). The bactericide and bacteriostatic role of the HBOT could be essential as an adjuvant therapy that may reduce the number of hospitalization days. In this regard, the therapy in question will induce two types of response: optimization of leukocyte function and bacterial destruction (Mader et al., 1980). In addition, synergism can occur in relation to antibiotics and may even have a bacteriostatic and bactericidal role when referring to anaerobic agents (Sousa, 2006). HBOT also plays a bacteriostatic role for Escherichia coli and Pseudomonas spp. This directly interferes with the toxemic activity resulting from bacterial proliferation (Edwards, 2010b; Mazzi, 2019). Approximately 76% (31/41) required a hospitalization period of more than 7 days requiring a therapy indicated for acute wounds which had difficult healing where hypoxia occurs, requiring diverse cellular phenomena, such as the proliferation of fibroblasts, collagen synthesis, and neo-epithelialization (Gill and Bell, 2004; Kulikovsky et al., 2009; Gomes and Jesus, 2012). The HBOT with a pressure equivalent to 3 ATA promotes a plasma oxygen concentration similar to 60 ml/l. Thus, breathing in an environment with 100% oxygen may help the ischemic tissue and potentiate the action of certain antibiotics, such as quinolones (Jones and Cooper, 2021), which was one of the antibiotics selected to the antibiotic therapy protocol at admission. This therapy is used as a safe adjuvant treatment for wound healing due to its complex, tight mechanisms that require a well-orchestrated interaction of molecular and cellular events. In ischemia-perfusion processes, HBOT results in an interface between neutrophils and endothelial cells, promoting arterial vasodilation and improving cellular damage (Baiula et al., 2020). The cellular effects of the HBOT are based on cellular changes that may enhance the up-regulation of oxidative stress [reactive oxygen species (ROS) generation; antioxidant suppression, such as catalase; pro-oxidant expression, such as inducible nitric oxide synthase]; lipid peroxidation (Gurer et al., 2006); and apoptosis (i.e., caspase) (Zhang et al., 2008; Migita et al., 2016). Also, in humans, the HBOT has shown to upregulate antioxidant gene expression in endothelial cells, protecting against oxidative damage (Francis and Baynosa, 2017). HBOT was considered a support therapy for the internal medicine protocol appropriate to each situation in this study. Cytology and culture associated with antibiogram should be carried out for greater success, although in our study only 39% (16/41) of the cases experienced it . As for the need for surgical cleaning, 54% (22/41) of Gd and Gc experienced this type of approach. This is common in human medicine in the treatment of difficult-to-heal ulcers, mainly in diabetes (Bhutani and Vishwanath, 2012; Everett and Mathioudakis, 2018; Liandro et al., 2020; Menezes et al., 2020). In all of the studies mentioned, the HBOT was considered beneficial in improving wound healing. In our study, no SEM was observed, thus with no occurrence of barotrauma or seizure process. However, during the compression and decompression phases, SEm appeared, such as 24% of swallowing effect, followed by 9% (4/41) of vocalization effect and 7% (3/41) of yawning effect. Also, it was verified that the acts of ear scratching and head shaking were not observed, possibly due to the protocol of admission to the study that required observation of the external auditory canal and tympanic membrane. This was not in accordance with Birnie et al. (2018) because, in his study, this procedure was not routinely carried out. The MVS was used in this pilot study as an adaptation and association of specific parameters of two human scales (VSS and Southampton Scale). This allowed the monitoring of 41 patients (25 dogs and 16 cats) with a homogenous and quantitative evaluation in regard to wound healing (Nguyen et al., 2015). Furthermore, all of these patients had infected wounds with a bad prognosis that were large and irregular, justifying the need and use of our scale. Our study observed, regarding the MVS classification, a decrease in relation to the average and median classification since the reduction between MVST0 and MVST1 was 2 points, decreasing from an average of 15–13. On the other hand, between MVST1 and MVST2, the decrease was more evident, having reduced 4 points, thus going from an average of 13–9. There was still a reduction between the MVST2 and MVST3 classification, not so evident, in about 2 points. This evolution was maintained for Gd and Gc. In the present study, the regression rate was 80% (33/41) regarding the difference between MVST0 and MVST4. This rate is identical to that observed by Mazzi (2019) that showed regression of 79.08% between the first and the sixth session. One reason for this evolution is possibly due to the peripheral vasoconstriction caused by the HBOT that promotes a faster reduction of the swelling tissues. The HBOT also has an angiogenic role, increasing neovascularization (Sousa, 2006; Edwards, 2010a; Gomes and Jesus, 2012) and reducing inflammation, that is present in the acute phase (Edwards, 2010b; Oyaizu et al., 2018) through the production of cytokines by the monocyte–macrophage complex (Zhang et al., 2008). Also, it allowed adequate blood flow, oxygen, and nutrients’ supply (Andrade and Santos, 2016). Neovascularization has been justified by the ability to produce fibroblast growth factors (Asano et al., 2007; Barbosa et al., 2020), both locally and systemically. ROS allows an increase in the production of growth factor VEGF and tissue growth factor-β (TGF-) (Lam et al., 2011; Francis and Baynosa, 2017). During the peripheral vasoconstriction process, there is an increase in systemic blood pressure and consequently a reduction in heart rate, which is a possible explanation for why 100% of the study’s population, in both Gd and in Gc, presented a relaxed behavior during HBOT without the need for sedation or anxiolytic drugs (Edwards, 2010b). In the present study, it was observed that dogs took longer to obtain MVS ratings indicative of a good prognosis compared to cats, thus justifying the strong statistical significance (X2 (1, N=41)=5.3, p=0.02) between species and hospitalization time. In addition, cats recovered within a lower number of sessions (X2(1, N=16)=0.01, p=0.01), since in a universe of eight cats, six were released in less than 7 days, suggesting that cats have an advantage over dogs. This phenomenon is possibly related to the lesion size compared to the total mass of the individual. There was also a trend of statistical significance (X2 (1, N=41)=3.6, p=0.05) between the time that elapsed from the injury to the admission since 25 patients who were admitted in a shorter time period were allowed to leave the hospital with a good MVS rating. In clinical practice, therapeutic guidelines are necessary and the present study suggested that the MVST1 had a strong significant statistical relationship with hospitalization time (X2 (2, N=41)=10.6, p=0.005). Therefore, extra importance to the MVS value in the first 24 hours (MVST1) may be suggested. It was also found that of the 20 patients with MVST1 > 10 and ≤ 15 (reserved prognosis), 18 required a longer period of hospitalization for 7 days, as well as out of 9 patients with MVST1 > 15 (poor prognosis), 8 patients needed to stay longer than 7 days. Thus, 76% of Gd and Gc (31/41) remained in the hospital for more than 7 days. Considering these results, we could suggest that HBOT was safe, for both Gd and Gc, and it may be a possible prescribed therapy in smaller animal practices. This pilot clinical study had several limitations. The main one was a lack of a control group due to its clinical context and ethical reasons. In addition, no objective evaluation method was used, such as mathematical computational methods that could better explain HBOT benefits. The present study should continue to clarify the referred therapy and its mode of action and apply the MVS as an assessment tool in wounds. Therefore, this may be a valuable tool in the recovery of critically ill patients that allows a greater probability for clinical success and reduction in limb amputations and euthanasia rates, thus requiring a homogenous, frequent, and quantitative monitorization with the MVS. We can then conclude that the hyperbaric chamber is a safe and low-risk intervention, with the adverse events being intermittent and typically not severe. It would be interesting, in relation to the hyperbaric chamber, to create guidelines in veterinary medicine and create a European Consensus Conference on HVM® . Conflict of interestThe authors declare that there is no conflict of interest. ReferencesAbdullah, M., Al-Waili, N., Butler, G. and Baban, N. 2006. Hyperbaric oxygen ad adjunctive therapy for bilateral compartment syndrome, rhabdomyolysis, and acute renal failure after heroin intake. Arch. Med. 37, 559–562. Andrade, S.M. and Santos, I.C.R.V. 2016. Oxigenoterapia hiperbárica para tratamento de feridas. Rev. Gaúcha. Enferm. 37, 1–7. Arikan, S., Adas, G., Barut, G., Toklu, A.S., Kocakusak, A. and Uzun, H. 2005. An evaluation of low molecular weight heparin and hyperbaric oxygen treatment in the prevention of intra-abdominal adhesions and wound healing. Am. J. Surg. 189, 155–160. Asano, T., Kaneko, E., Shinozaki, S., Imai, Y., Shibayama, M., Chiba, T., Ai, M., Kawakami, A., Asaoka, H., Nakayama, T., Mano, Y. and Shimokado, K. 2007. Hyperbaric oxygen induces basic fibroblast growth factor and hepatocyte growth factor expression, and enhances blood perfusion and muscle regeneration in mouse ischemic hind limbs. Circ. J. 71, 405–411. Baiula, M., Greco, R., Ferrazzano, L., Caligiana, A., Hoxha, K., Bandini, D., Longobardi, P., Spampinato, S. and Tolomelli, A. 2020. Integrin-mediated adhesive properties of neutrophils are reduced by hyperbaric oxygen therapy in patients with a chronic non-healing wound. PLoS One 18, 1–22. Barbosa, P.R., Gurgel, L., Araujo, P. and Silva, V. 2020. Hyperbaric oxygen therapy in the wound healing process: literature review. Rev. Enferm. Atual. Derme. 93, 20–31. Bhutani, S. and Vishwanath, G. 2012. Hyperbaric oxygen and wound healing. Indian J. Plast. Surg. 45, 316–324. Bilic, I., Petri, N.M., Bezic, J., Alfirevic, D. and Modun, D. 2005. Effects of hyperbaric oxygen therapy on experimental burn wound healing in rats: a randomized controlled study. Undersea Hyperb. Med. 32, 1–9. Birnie, G.L., Fry, D.R. and Best, M.P. 2018. Safety and tolerability of hyperbaric oxygen therapy in cats and dogs. J. Am. Anim. Hosp. Assoc. 54, 188–194. Bitterman, H. 2009. Bench-to-bedside review: oxygen as a drug. Crit. Care 13, 203–205. Fearmonti, R., Bond, J., Erdmann, D. and Levinson, H. 2010. A review of scar scales and scar measuring devices. ePlasty 10, 354–362. Choudhury, R. 2018. Hypoxia and hyperbaric oxygen therapy: a review. Int. J. Gen. Med. 11, 431–442. Çimşi, M., Uzun, G. and Yildiz, S. 2009. Hyperbaric oxygen therapy as an anti-infective agent. Expert Rev. Anti Infect. Ther. 7, 1015–1026. Edwards, M.L. 2010a. Hyperbaric oxygen therapy. Part 1: history and principles. J. Vet. Emerg. Crit. Care 20, 284–288. Edwards, M.L. 2010b. Hyperbaric oxygen therapy - part 2: application in disease. J. Vet. Emerg. Crit. Care 20, 289–297. Everett, E. and Mathioudakis, N. 2018. Update on the management of diabetic foot ulcers. Ann. N. Y. Sci. 141, 153–165. Ferreira, A., Barbieri, C., Mazzer, N., Campos, A. and Mendonça, A. 2008. Mensuração de área de cicatrização por planimetria após aplicação do ultra-som de baixa intensidade em pele de rato. Braz. J. Phys. Ther. 12, 351–358. Francis, A. and Baynosa, R. 2017. Ischemia-reperfusion injury and hyperbaric oxygen pathways: a review of cellular mechanisms. Diving Hyperbar. Med. 47, 110–117. Gesell, L.B. 2008. Hyperbaric oxygen therapy indications. Undersea Hyperbar. Med. Soc. 12, 4–8. Gill, A.L. and Bell, C.N.A. 2004. Hyperbaric oxygen: Its uses, mechanisms of action, and outcomes. QJM 97, 385–395. Gomes, C. and Jesus, C. 2012. Benefícios da aplicação da oxigenoterapia hiperbárica na cicatrização de feridas das extremidades inferiores. J. Aging Inov. 12, 40–47. Gottrup, F., Melling, A. and Hollander, D. 2005. An overview of surgical site infections: etiology, incidence and risk factors. EWMA J. 5, 11–15. Gurer, A., Ozdogan, M., Gomceli, I., Demirag, A., Gulbahar, O., Arikok, T., Kulacoglu, H., Dundar, K. and Ozlem, N. 2006. Hyperbaric oxygenation attenuates renal ischemia-repurfusion injury in rats. Transplant. Proc. 38, 3337–3340. Handley, M. and Cooper, J. 2017. Chronic refractory osteomyelitis. Treasure Island, FL: StatPearls. vol. 3, pp: 4–8. Hengel, T.V., Haar, G.T. and Kirpensteijn, J. 2013. Wound management: a new protocol for dogs and cats. In Reconstructive surgery and wound management of the dog and cat. Boca Raton, FL:CRC Press, pp: 21–48. Jokinen-Gordon, H., Barry, R., Watson, B. and Covington, S. 2017. A retrospective analysis of adverse events in hyperbaric oxygen therapy (2012-2015): lessons learned from 1.5 million treatments. Adv. Skin Wound Care 3, 125–129. Jones, M. and Cooper, J. 2021. Hyperbaric therapy for wound healing. Treasure Island, FL: StatPearls, vol. 18, pp: 1–8. Kirby, J.P., Snyder, J., Schuerer, D.J.E., Peters, J.S. and Bochicchio, G.V. 2019. Essentials of hyperbaric oxygen therapy: 2019 review. Mo. Med. 116, 176–179. Klemetti, E., Rico-Vargas, S. and Mojon, P. 2005. Short duration hyperbaric oxygen treatment affects blood flow in rats: pilot observations. Lab. Anim. 39, 116–121. Knighton, D.R., Halliday, B. and Hunt, T.K. 1984. Oxygen as an antibiotic: the effect of inspired oxygen on infection. Arch. Surg. 119, 199–204. Kulikovsky, M., Gil, T., Mettanes, I., Karmeli, R. and Har-Shai, Y. 2009. Hyperbaric oxygen therapy for non-healing wounds. Isr. Med. Assoc. J. 11, 480–485. Lam, N.A., Demaria, M., Stanley, B.J., Hauptman, J.G., Steficek, B.A., Fritz, M.C., Ryan, J.M., Moore, T.W. and Hadley, H.S. 2011. Effects of negative pressure wound therapy on healing of open wounds in dogs. Vet. Surg. 40, 658–669. Liandro, C.L., Santos, M., Carreiro, M., Cunha, K. and Paula, D. 2020. Oxigenoterapia hiperbárica como tratamento adjuvante para feridas: estudo de prevalência. Enferm. Foco. 11, 29–34. Mader, J., Brown, G. and Guckian, J. 1980. A mechanism for the amelioration by hyperbaric oxygen of experimental staphylococcal osteomyelitis in rabbits. J. Infect. Dis. 142, 915–922. Mazzi, M.F. 2019. A utilização da oxigenoterapia hiperbárica no processo de cicatrização por mordedura em cão. Pubvet. 12, 239–250. Mazzi, M.F. 2018. A utilização da oxigenoterapia hiperbárica no tratamento de fasceíte necrotizante do prepúcio de um cão idoso. Pubvet. 12, 1–7. Memar, M.Y., Yekani, M., Alizadeh, N. and Baghi, H.B. 2019. Hyperbaric oxygen therapy: antimicrobial mechanism and clinical application for infections. Biomed. Pharmacother. 109, 440–447. Menezes, E.O., Cintra, B. and Félix, V. 2020. Utilização da oxigenoterapia hiperbárica no tratamento da doença vascular periférica: uma revisão sistemática. REAS/EJCH. 12, 1–11. Migita, H., Yoshitake, S., Tange, Y., Choijookhuu, N. and Hishikawa, Y. 2016. Hyperbaric oxygen therapy suppresses apoptosis and promotes renal tubular regeneration after renal ischemia/reperfusion injury in rats. Nephrourol. Mon. 8, e34421. Muhonen, A., Haaparanta, M., Gronroos, T., Bergman, J., Jnuuti, J., Hinkka, S. and Happonen, R.P. 2004. Osteoblastic acvtivity and neoangiogenesis in the distracted bone of irradiated rabbit mandible with or without hyperbaric oxygen treatment. Int. J. Oral Maxillofac. Surg. 33, 173–178. Nguyen, T., Feldstein, S., Krakowski, A. and Shumaker, P. 2015. A review of scar assessment scales. Semin. Cutan. Med. Surg. 34, 28–36. Oyaizu, T., Enomoto, M., Yamamoto, N., Tsuji, K., Horie, M., Muneta, T., Sekiya, I., Okawa, A. and Yagishita, K. 2018. Hyperbaric oxygen reduces inflammation, oxygenates injured muscle, and regenerates skeletal muscle via macrophage and satellite cell activation. Sci. Rep. 8, 1–12. Pavletic, M.M. 2018. In Atlas of small animal wound management and reconstructive surgery, 3rd ed. Hoboken, NJ: John Wiley & Sons, Inc, pp: 120–128. Roberto, P. and Barbosa, A. 2019. Oxigenoterapia hiperbárica no processo de cicatrização de feridas: revisão de literatura. Rev. Enferm. Atual. Derme. 24, 1–8. Shah, J. 2010. Hyperbaric oxygen therapy. J. Am ColCertif. Wound Spec. 2, 9–13. Sheffield, P.J. and Smith, A. 2002. Physiological and pharmacological basis of hyperbaric oxygen therapy. In Hyperbaric surgery: perioperative care. Best Publishing, San Antonio, Texas, USA, pp. 63–104. Sousa, J.G. 2006. A medicina hiperbárica: uma especificidade da medicina naval. Rev. Militar. 7, 1–17. Zamboni, W.A., Browder, L.K. and Martinez, J. 2003. Hyperbaric oxygen and wound healing. Clin. Plast. Surg. 30, 67–65. Zhang, Q., Chang, Q., Cox, RA., Gong, X. and Gould, LJ. 2008. Hyperbaric oxygen attenuates apoptosis and decreases inflammation in an ischaemic wound model. J. Invest. Dermatol. 128, 2102–2112. Zhang, T., Gong, W., Li, Z., Yang, S., Zhang, K. and Yin, D. 2007. Efficacy of hyperbaric oxygen on survival of random pattern skin flap of diabetic rats. Undersea Hyperb. Med. 34, 335–339. | ||
| How to Cite this Article |
| Pubmed Style Bimbarra S, Gouveia D, Carvalho C, Cardoso A, Gamboa O, Ferreira A, Teixeira R, Martins A. Effects of hyperbaric oxygen therapy on wound healing in veterinary medicine: a pilot study. Open Vet. J.. 2021; 11(4): 544-554. doi:10.5455/OVJ.2021.v11.i4.4 Web Style Bimbarra S, Gouveia D, Carvalho C, Cardoso A, Gamboa O, Ferreira A, Teixeira R, Martins A. Effects of hyperbaric oxygen therapy on wound healing in veterinary medicine: a pilot study. https://www.openveterinaryjournal.com/?mno=85239 [Access: January 25, 2026]. doi:10.5455/OVJ.2021.v11.i4.4 AMA (American Medical Association) Style Bimbarra S, Gouveia D, Carvalho C, Cardoso A, Gamboa O, Ferreira A, Teixeira R, Martins A. Effects of hyperbaric oxygen therapy on wound healing in veterinary medicine: a pilot study. Open Vet. J.. 2021; 11(4): 544-554. doi:10.5455/OVJ.2021.v11.i4.4 Vancouver/ICMJE Style Bimbarra S, Gouveia D, Carvalho C, Cardoso A, Gamboa O, Ferreira A, Teixeira R, Martins A. Effects of hyperbaric oxygen therapy on wound healing in veterinary medicine: a pilot study. Open Vet. J.. (2021), [cited January 25, 2026]; 11(4): 544-554. doi:10.5455/OVJ.2021.v11.i4.4 Harvard Style Bimbarra, S., Gouveia, . D., Carvalho, . C., Cardoso, . A., Gamboa, . O., Ferreira, . A., Teixeira, . R. & Martins, . A. (2021) Effects of hyperbaric oxygen therapy on wound healing in veterinary medicine: a pilot study. Open Vet. J., 11 (4), 544-554. doi:10.5455/OVJ.2021.v11.i4.4 Turabian Style Bimbarra, Sara, Debora Gouveia, Carla Carvalho, Ana Cardoso, Oscar Gamboa, Antonio Ferreira, Rute Teixeira, and Angela Martins. 2021. Effects of hyperbaric oxygen therapy on wound healing in veterinary medicine: a pilot study. Open Veterinary Journal, 11 (4), 544-554. doi:10.5455/OVJ.2021.v11.i4.4 Chicago Style Bimbarra, Sara, Debora Gouveia, Carla Carvalho, Ana Cardoso, Oscar Gamboa, Antonio Ferreira, Rute Teixeira, and Angela Martins. "Effects of hyperbaric oxygen therapy on wound healing in veterinary medicine: a pilot study." Open Veterinary Journal 11 (2021), 544-554. doi:10.5455/OVJ.2021.v11.i4.4 MLA (The Modern Language Association) Style Bimbarra, Sara, Debora Gouveia, Carla Carvalho, Ana Cardoso, Oscar Gamboa, Antonio Ferreira, Rute Teixeira, and Angela Martins. "Effects of hyperbaric oxygen therapy on wound healing in veterinary medicine: a pilot study." Open Veterinary Journal 11.4 (2021), 544-554. Print. doi:10.5455/OVJ.2021.v11.i4.4 APA (American Psychological Association) Style Bimbarra, S., Gouveia, . D., Carvalho, . C., Cardoso, . A., Gamboa, . O., Ferreira, . A., Teixeira, . R. & Martins, . A. (2021) Effects of hyperbaric oxygen therapy on wound healing in veterinary medicine: a pilot study. Open Veterinary Journal, 11 (4), 544-554. doi:10.5455/OVJ.2021.v11.i4.4 |