| Review Article | ||
Open Vet. J.. 2022; 12(4): 525-539 Open Veterinary Journal, (2022), Vol. 12(4): 525–539 Review Article Paratuberculosis control strategies in dairy cattle: A systematic reviewBrahian Camilo Tuberquia-López, Nathalia M Correa-Valencia, Miguel Hernández-Agudelo, Jorge A Fernández-Silva and Nicolás Fernando Ramírez-Vásquez*Centauro, Escuela de Medicina Veterinaria, Facultad de Ciencias Agrarias, Universidad de Antioquia UdeA, Medellín, Colombia *Corresponding Author: Nicolás Fernando Ramírez-Vásquez. Centauro, Escuela de Medicina Veterinaria, Facultad de Ciencias Agrarias, Universidad de Antioquia, Colombia. Email: nicolas.ramirez [at] udea.edu.co Submitted: 28/02/2022 Accepted: 14/07/2022 Published: 12/08/2022 © 2022 Open Veterinary Journal
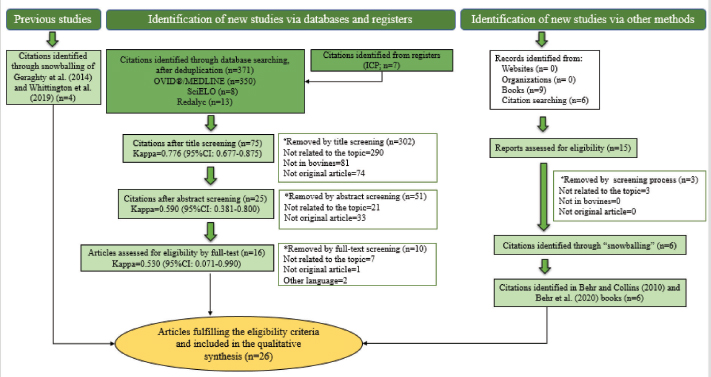
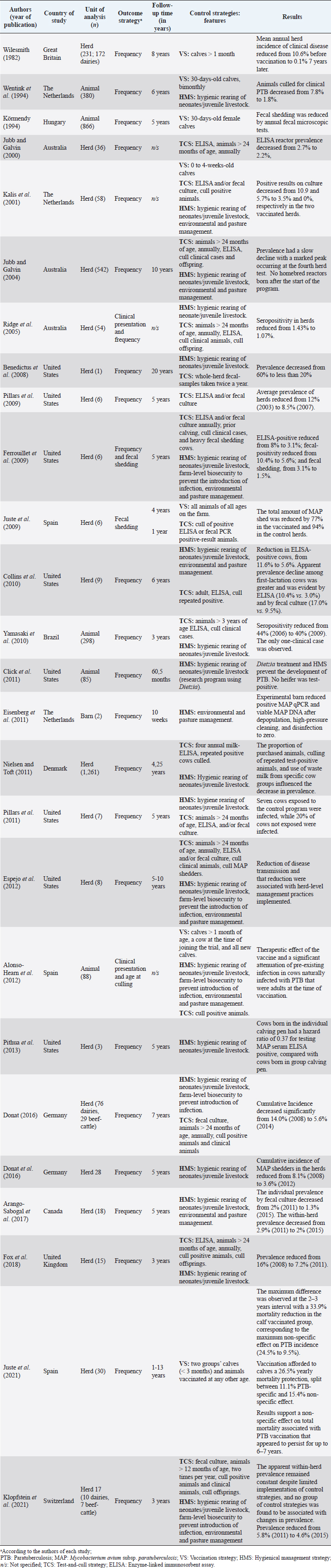
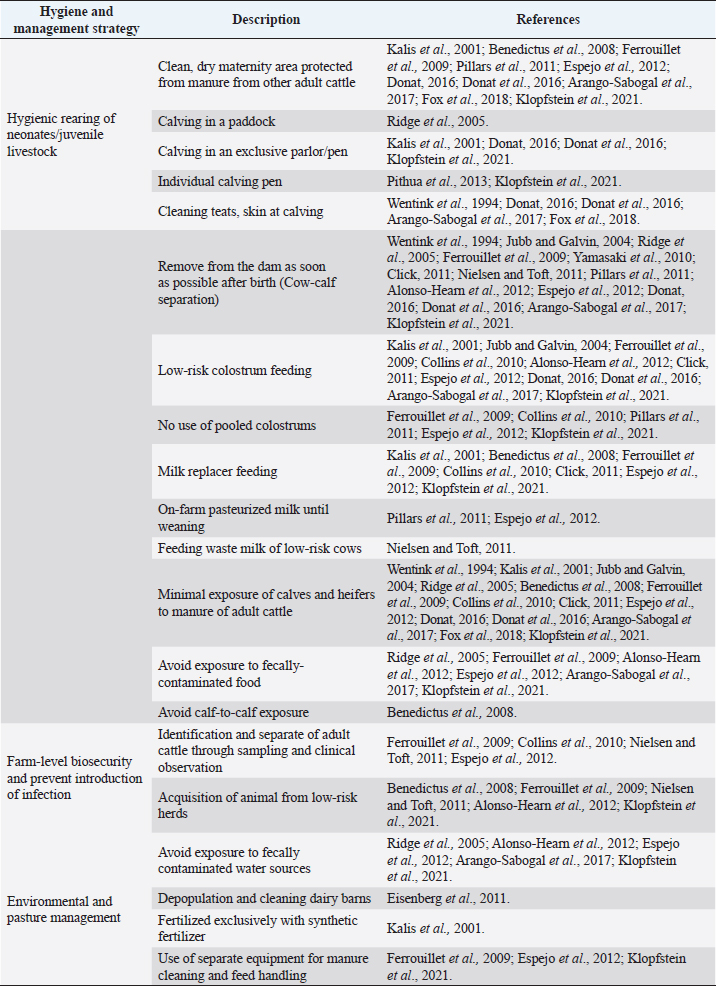
AbstractBackground: Mycobacterium avium subsp. paratuberculosis is the causative agent of paratuberculosis (PTB), incurable enterocolitis, affecting domestic and wild ruminants. Economic losses, impacts on animal health and welfare, and public health concerns justify its herd-level control. Aim: To systematically collect information to answer: What are the control and eradication strategies of PTB in dairy cattle worldwide? Methods: The search procedure was carried out on October 2nd, 2019, and updated on August 3rd, 2021, using OVID®, SciELO, and Redalyc databases, and the registers from the International Colloquium on Paratuberculosis (1991–2018). The inclusion criteria considered articles published in English, Portuguese, and Spanish and in peer-reviewed journals. The exclusion criteria included irrelevant topics, species other-than bovines, and not original articles. Definitive studies were obtained through the consensus of the authors on eligibility and quality. Data extraction was performed, considering bibliographic information, control and outcome strategies, follow-up time, and results. Results: Twenty-six relevant studies were found, reporting the use of three grouped control strategies: hygiene and management strategy (HMS), test-and-cull strategy (TCS), and vaccination strategy (VS). The HMS was the most common one (20/26), followed by TCS (17/26) and VS (7/26). Combined control strategies such as TCS-HMS (12/26), TCS-VS (1/26), and HMS-VS (1/26) were also described, and the consideration of the three control strategies (TCS-HMS-VS) was reported in two articles. The HMS included practices such as neonates/juvenile livestock hygiene, biosecurity, prevention of infection introduction into the herd, and environmental management. Within HMS, the most frequent practices were to remove calves from their dams as soon as possible after birth and to keep the minimal exposure of calves and heifers to adult cattle. As limitations, within the HMS, it is considered that some strategies cannot be included due to lack of compliance, or the application of the same strategy among one study and another may have a different degree of interpretation; publication bias was not controlled since the results of the control programs in endemic countries may be not available. Conclusion: The main PTB control strategies in dairy cattle worldwide are HMS, TCS, and VS. The use of one or several combined strategies has been found to succeed in controlling the disease at the herd-level. Keywords: Control strategy, Dairy cattle, Eradication, Johne’s disease. IntroductionParatuberculosis (PTB), also known as Johne’s disease, is a severe slow-developing and incurable granulomatous enteritis caused by Mycobacterium avium subsp. paratuberculosis (MAP) (Clarke, 1997). This disease affects cattle and other domestic and wild ruminants (Sweeney et al., 2012). A localized infection is the first stage of the disease, lately resulting in chronic granulomatous enteritis with diarrhea, weight loss, and, finally, death (Clarke, 1997). Several tests are available for the ante-mortem detection of MAP-infected animals, including the detection of MAP antibodies, DNA, or live organisms by the culture. The diagnostic tests available are imperfect although useful if applied properly when a specific purpose has been identified (Nielsen and Toft, 2008). This worldwide-extended disease affects more than 50% of herds in countries with a significant dairy industry (Manning and Collins, 2010). Economic losses are higher in PTB-infected herds, due to reduced milk yield, increased cow-heifer replacement costs, lower cull-cow revenue, and greater cow mortality (Hutchinson, 1996; Ott et al., 1999; Lombard et al., 2005; Gonda et al., 2007; Richardson and More, 2009; Smith et al., 2010). On the other hand, MAP has been associated with Crohn’s disease (Feller et al., 2007) and other human autoimmune diseases, such as Blau syndrome, type I diabetes, Hashimoto thyroiditis, and multiple sclerosis, reinforcing the zoonotic potential of this pathogen (Lee et al., 2011; Sechi and Dow, 2015). Hence, MAP primary public health concerns are related to food and environmental contamination (Eltholth et al., 2009). Therefore, due to its direct effects on animal health, economic losses, potential public health implications, and livestock trade, PTB is listed by the World Organization for Animal Health. Geraghty et al. (2014) conducted a narrative review on PTB control programs in six endemic countries, reporting a significant heterogeneity among them. More recently, Whittington et al. (2019) conducted a narrative review on 48 countries (2012–2018) on the same topic. Authors reported that 20% of the herds of half of the countries of study were MAP-infected. In addition, PTB report is mandatory in most of the countries, and only 46% (22/48) had an established control program for the disease. Animal health and production losses were found to be the rationale for the control programs in these countries, and the most common objective was to reduce PTB prevalence. PTB herd-level control is difficult due to its long incubation period, imperfect diagnostic tests, and persistent environmental survival (Kennedy and Benedictus, 2001). To reduce the risk of infection, control strategies should aim to eliminate infected animals (particularly those affected and infectious from the herd—e.g., test-and-cull strategy; TCS), to break down transmission routes of the disease, and to reduce the risk of infection, particularly to young animals (Johnson-Ifearulundu and Kaneene, 1998; Garry, 2011). One of the main interventions reported in dairy cattle is to avoid the contact of calves with feces of adult cattle (Doré et al., 2012), interfering with the fecal-oral transmission. Other practices include feeding pasteurized milk or colostrum from MAP-seronegative cows and calf and heifer-hygienical raising strategies (Aly et al., 2015). These last are known as hygiene and management strategies (HMS). On the other hand, vaccination strategies (VS) are reported to reduce the clinical incidence of PTB, delaying the onset of the disease and reducing fecal shedding of MAP, thus reducing the economic losses and transmission of the disease (Bastida and Juste, 2011). Nevertheless, VS are controversial due to its possible interference with tuberculosis control programs (Coad et al., 2013; Serrano et al., 2017). Although some reports on PTB control strategies have been published, there is great variability in control strategies reported regarding their application within control programs in PTB endemic countries and the success in controlling the disease at the herd-level. Many of these control strategies have been implemented under different field conditions and they vary depending on the prevalence of diseases, the diagnostic strategies, the control objective, and the sanctions for non-participation. A systematic review (SystRev) about the control strategies that have been implemented for the control of PTB in dairy cattle would provide a great opportunity to understand the best opportunity to learn from past and collective experiences of PTB control and to design and implement improved control programs in the future. Therefore, we aimed to systematically collect information on the control strategies of PTB in dairy cattle worldwide. Materials and MethodsSystRev was designed, performed, and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, suggested by Page et al. (2021). An a priori established and pre-tested SystRev protocol was carried out, including the study question, procedure for literature search, study inclusion/exclusion criteria and checklists for conducting relevance screening, basic characterization, methodological assessment, and data extraction on relevant primary research. Search strategyThe identification of relevant articles considered a specific research question: What are the control and eradication strategies of PTB in dairy cattle worldwide? The search procedure was performed on October 2, 2019 and updated on August 3, 2021. The question was divided into components and the search terms used to find relevant studies in the platforms were [(control OR management OR regulation?) AND (eradication OR elimination OR clearance) AND (paratuberculosis OR johne* disease? OR mycobacterium avium paratuberculosis) AND (dairy OR cow? OR livestock OR cattle OR bovi* OR ruminant* OR calf OR calve? OR heifer? OR bull? OR steer?)]. Three search databases (i.e., OVID®/MEDLINE, SciELO, Redalyc). The registers from the proceedings of the 3rd (1991) to the 12th (2014) International Colloquium on Paratuberculosis (ICP), were available from the platforms explored. The 13th and 14th ICP proceedings (2016 and 2018, respectively) were available at the International Association for Paratuberculosis web site (http://www.paratuberculosis.net/). This last material was hand-searched for existing published primary studies. Finally, references related to the SystRev subject were hand-searched in Behr and Collins (2010) and Behr et al. (2020) books to track primary publications. Eligibility criteriaThe inclusion criteria considered only articles published in English, Portuguese, and Spanish and in peer-reviewed journals. Findings were not limited by year or country of publication. The first selection of citations was done according to the information contained only in the title. Two of the authors completed the selection and a kappa coefficient was estimated. The exclusion criteria were: i) irrelevant topics (e.g., Crohn's disease, economic impact, Mycobacterium bovis, diagnosis, and modeling); ii) species other-than bovines (e.g., goats, sheep, human); iii) not an original article (e.g., review, book). Duplicated articles were not considered. All citations selected by at least one of the two authors were considered eligible to continue in the process. The eligible citations were screened by two of the authors using the abstract. A kappa coefficient was estimated. Exclusion criteria were the same as for the title screening. Conflicts were resolved through consensus between authors and if necessary, a third author was consulted. The full text of selected articles was reviewed by two authors to identify and extract relevant information to answer the research question. Each full text was reviewed with particular attention to the materials and methods and results sections. A kappa coefficient was estimated. Articles were considered eligible using the same exclusion criteria described above. Conflicts were resolved through consensus between authors and if necessary, a third author was consulted. Two of the authors hand-searched the reference lists of relevant articles identified by the full-text screening for additional published primary articles (“snowballing” procedure). In addition, the same strategy was applied to two literature reviews on the topic (Geraghty et al., 2014; Whittington et al., 2019). The ICP proceedings and other abstracts identified during the primary search were revised to identify further citations in peer-reviewed journals. In this concern, abstracts found able to answer the research question were identified and an email was sent to the corresponding author to inquire if the abstract was furtherly published in a peer-reviewed journal. The articles obtained from this previous step, as well as those detected at the Behr and Collins (2010) and Behr et al. (2020) books, were screened by two of the authors. Data extraction and descriptive statisticsAfter all available articles were compiled, data extraction was performed by one of the authors, considering bibliographic information, control strategies—categorized in TCS, HMS, and VS, according to Bastida and Juste (2011), outcome strategies (e.g., prevalence, incidence, test-positivity, and fecal shedding rate), follow-up time of the program, and results to the interventions. A second author reviewed data-extraction products. ResultsThe combined results from the search platforms yielded 371 eligible citations (after deduplication), potentially related to the subject of this SystRev. The review of the reference lists in the Behr and Collins (2010) and Behret al. (2020) books provided six eligible citations. The hand searching of the ICP proceedings (3rd to 14th) delivered seven eligible citations, of which none continued in the process, since they were not furtherly published in peer-reviewed journals (according to the email responses by the corresponding authors). Therefore, the final number of citations was 377. After reading the titles of the articles, 302 were considered irrelevant (agreed by the two authors). The final number of citations based on title screening was 75 (retained by at least one of the authors). After reading the abstracts of the articles, 51 were excluded and 25 original articles remained for the full-text review (by both authors). Ten were excluded because of the criteria already described by title and abstract screening. The full text of 16 articles were completely reviewed and kept for data extraction. The “snowballing” strategy was then applied through the reference lists of the 16 definitive articles and six more citations were found. In addition, the same strategy was applied to two literature reviews (Geragthy et al., 2014; Whittington et al., 2019) and four more citations were found. The final number of articles full filling the eligibility criteria and hence included in the qualitative synthesis was 26. Figure 1 describes the review protocol and the selection of relevant articles. Twenty-six selected articles were published in 15 different journals, all in English, except for one in Portuguese. The relevant articles were published between 1982 and 2021. Most of the articles (50%; 13/26) were published between 2008 and 2013 and the United States was the most common country of publication (50%; 13/26), followed by The Netherlands (15%; 4/26), Australia (7%; 2/26), Spain (7%; 2/26), Germany (7%; 2/26), Hungary (7%; 2/26), United Kingdom (7%; 2/26), Brazil (4%; 1/26) and Denmark (4%; 1/26). The control strategies were grouped into HMS, TCS, and VS. The HMS were the most common (77%; 20/26), followed by TCS (65%; 17/26), and VS (27%; 7/26). Combined control strategies such as TCS-HMS (46%; 12/26), TCS-VS (4%; 1/26), and HMS-VS (4%; 1/26) were also described, and the consideration of the three control strategies (TCS-HMS-VS) was reported by two articles. The 77% (20/26) of the reported studies considered a range of 1–1,261 herds. A 26% (5/26) reported animal-level control strategies (ranging from 85 to 866 animals). Selected articles reported a follow-up time of 2.5 months to 20 years, mainly a 5-year follow-up (35%; 9/26). All selected articles found a reduction in their outcome strategies or expected results (e.g. prevalence, incidence, test-positivity, fecal shedding rate). The HMS were grouped into practices used in control programs for PTB as reported by Whittington et al. (2019): hygienic rearing of neonates/juvenile livestock (if hygiene actions were indicated in the calving area, management of new-borns, calves and heifers), herd-level biosecurity, prevention of infection introduction (related to actions to identify infected and infectious animals and the purchase of external animals), and environmental and pasture management (related to actions to avoid contamination by MAP). Within HMS, the most common practices were to remove calves from their dams as soon as possible after birth (14/20) and to limit the exposure of calves and heifers to adult cattle manure (14/20). Detailed information extracted from the 26 relevant articles describing the PTB control strategies in dairy cattle is shown in Table 1. Table 2 describes the HMS reported in the selected articles and the corresponding management practice.
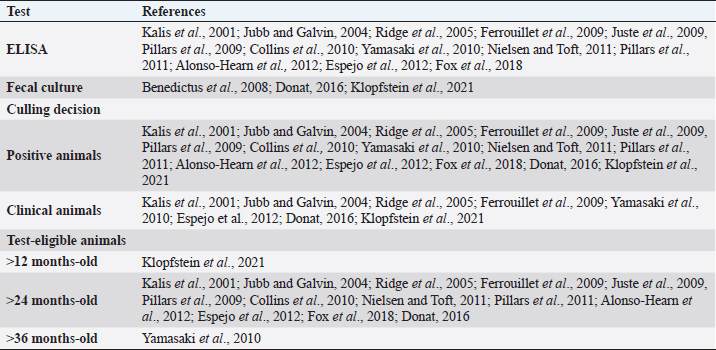
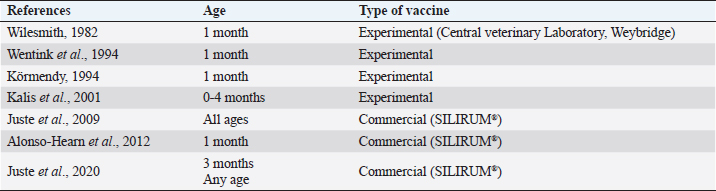
Fig. 1. Flow-chart of selection of relevant articles (PRISMA), describing the progress of the studies through the SystRev. Within TCS, enzyme-linked immunosorbent assay (ELISA) was used in 13/17 selected publications on this specific strategy, fecal culture was reported in three articles. Other practices included culling positive animals (13/17) and culling clinical ones (6/17), and culling decisions included to cull offspring and fecal shedders. All tests were performed in animals > 24 months of age, except for one article, which considered animals > 3 years (Yamasaki et al., 2010). No testing-frequency was found, but it was reported that most of the tests were carried out on a year-basis (5/17). Table 3 describes the TCS reported in the selected articles. Articles on VS reported animals of all ages, but mostly >1-month-old. Three studies reporting this strategy used one commercial vaccine (SILIRUM Paratuberculosis®. CZ Veterinaria S.A., Pol. La Relva, Torneiros, Spain) and four experimental approaches, all used heat-inactivated bacterin of MAP. According to all reports, the VS is important for reducing the fecal excretion of MAP and the clinical presentation of PTB. Table 4 describes seven references reporting VS. DiscussionStudies about PTB control strategies in dairy cattle were reviewed using a systematic methodology for the first time. Our purpose was to compile all published available evidence about control strategies for the disease, considering different practices and strategies applied along the reviewed studies. Our findings allowed us, not only to answer the specific research question, but to present other elements of the control programs, such as frequency strategies, follow-up times, and outcomes. As expected, the HMS were the most reported strategies throughout the selected studies, showing a wide variety of corrective actions, which are supported in the different observational studies on MAP-transmission risk factors (Obasanjo et al., 1997; Johnson-Ifearulundu and Kaneene, 1999; Wells and Wagner, 2000; Caldow et al., 2001, Doré et al., 2012; Puerto-Parada et al., 2018). This may explain that most of the HMS applied in bovines are related to the protection against infection at susceptible ages (new-borns and calves under 12 months), such as the elimination/control of infection sources as feces, milk, and colostrum (Stabel, 2008; Pithua et al., 2009). Fourteen management strategies in this regard were found, representing the greatest control actions against PTB in dairy cattle. The purchase of external animals without history of MAP diagnosis has also been identified as a risk factor for the disease entry in a herd, even under control programs (Pillars et al., 2009; Correia- Gomes et al., 2010; Künzler et al., 2014; Pieper et al., 2015, Puerto-Parada et al., 2018); therefore, the management of closed herds, and the purchase of animals from farms with a known MAP-status is a recommended practice for dairy farmers. Although the application of all HMS is not mandatory, the risk assessment and management plans (Garry, 2011) —considered as the best methodology for PTB control programs, was reported in some of the relevant studies, with the disadvantage of hindering the structuring of comparable strategies and units of analysis (herd/animal-level) among studies. On the other hand, the different production systems (e.g. tie-stall, free-stall, grazing-based) represent a variety of production practices and models that make it difficult to standardize risk factors among herds in different regions or countries. Although many of the HMS have greater application to tie- or free-stall dairy systems, some strategies —such as fertilization with synthetic fertilizer-only, calving in a paddock, and the use of separate equipment for manure cleaning and feed handling, are of interest in dairy production systems such as grazing-based one. Table 1. Information on control strategies for paratuberculosis in dairy cattle, extracted from the selected studies (n=26) of the SystRev.
Table 2. Description of HMS for paratuberculosis control in dairy cattle, extracted from the selected studies (n=26) of the SystRev.
Table 3. Description of TCS for paratuberculosis control in dairy cattle, extracted from the selected studies (n =26) of the SystRev.
Table 4. Description of VS for paratuberculosis control in dairy cattle, extracted from the selected studies (n =26) of the SystRev.
The commitment of owners and herd managers during the long implementation time that demands PTB control, represents an important limitation. The participation of the producers and the perception of the importance of PTB in dairy production must be considered, since the first results can only be observed 1 to 2 years after the implementation of control strategies. Roche et al. (2019) carried out a study on the reasons why many Canadian producers did not want to continue in the PTB control programs. These authors found that producers tended to prioritize control of the disease on their farms based on previous experiences with the disease, in addition to limited visualization of benefits or the existence of official sanctions or regulations. The TCS of high-risk animals (e.g. affected, infectious animals) was also found as a control strategy for PTB. Culling positive animals that may develop clinical disease in the future —acting as a source of infection for the herd, is considered as a critical-point for the success of control programs. It is important to mention that no homogeneity was found among the studies for the definition of a test-positive case with respect to each diagnostic test used. ELISA is reported as the most widely used one (mainly because of its cost, ease, and time to perform), with a main disadvantage in terms of low sensitivity (Se) in subclinical animals (7%–15%) (Gilardoni et al., 2012). This fact can be controlled when serial screenings are performed, and decisions are made regarding the results of the diagnostic tests. A more sensitive test must be considered, aiming to detect most of the infected dams before drying-off. This practice could allow the infected dams to be managed separately and their colostrum and milk to be classed as high risk, reducing the chances of their calves (and others born in the calving pen) from becoming infected. We hypothesized that, despite the low Se of ELISA for the diagnosis of MAP, when the diagnostic purpose is considered (detection of infected, infectious, affected animals; Nielsen and Toft, 2008), and combined with HMS in a long-term control program, the success of the control program is expected to be greater. The few reports regarding the use of vaccination were an expected result, since some authors have reported variable fallouts around the different studies (Patton, 2011). Nevertheless, MAP-vaccination is an important strategy in reducing contamination risks by this pathogen and reducing or delaying economic losses and clinical effects, without fully preventing infection (Bastida and Juste, 2011). Bovine tuberculosis diagnosis-interference when using immunological tests is one of the main reasons for not vaccinating cattle. Some studies at the control program level have only reported its use in seven countries (Whittington et al., 2019). It is important to note that the use of vaccination has been reported as a successful strategy in the control of PTB in small ruminants (Reddacliff et al., 2006). Our findings support that VS is an interesting control strategy, specifically, to control MAP, since it shows a great advantage in preventing pathological and productive effects in dairy farms. Combining control strategies have shown better results to specific control objectives, such as decreasing prevalence in herds (Whittington et al., 2019). Specifically, TCS and HMS have been assessed in theoretical and mathematical models that simulate the transmission and control conditions of MAP in dairy farms (Marcé et al., 2010). Recently, Camanes et al. (2018) reported the results of a study on coupling population and infection dynamics. Authors suggested that herd-level relevant control strategies mainly depend on initial prevalence. In addition, a reduced calf exposure was confirmed to be the most effective strategy, followed by test frequency and the detected-and-culled infected-animals proportion. Similarly, Konboon et al. (2018), suggested that a combination of test-and-cull with a frequent manure removal was the most effective strategy in reducing incidence and prevalence and the risk of MAP occurrence. Remarkably, other control strategies reported by the literature (i.e. limiting calf-adult cow contacts, raising calves in a disease-free herd or colostrum management) were less effective. The prolonged time of application of control strategies is a fact for diseases with chronic behavior, as has been reported in other ones in cattle such as bovine tuberculosis (Palmer and Waters, 2011). Although the application of the control strategies is reported for an extensive period, it is important to mention that the complete eradication (or at least to present a low prevalence) of PTB is a scarcely reported experience, mainly followed in countries with high economic infrastructure, and, as Norway and Sweden (Whittington et al., 2019), where animal health efforts are focused on disease surveillance. Disease follow-up times should be surveyed with the appropriate use of diagnostics tests that allow the success of control strategies to be reported. Measuring the effect of control strategies based on some frequency strategies (e.g., prevalence, frequency, and positivity) was not accomplished due to lack of comparability among selected studies. The strengths of the present SystRev are a well-defined protocol, based on a recognized one (PRISMA statement), a clearly stated and delimited research question; we performed a comprehensive search from several databases and sources to identify studies, including general-purpose databases, search engines, journals, conference proceedings, book chapters, and books from 1910 (CAB Abstracts) to the date; and we assessed the eligibility of the studies by using pre-established and explicit exclusion criteria all along the process. No geographic or temporal constraints were considered, so no biases-related results are thought to be yielded. Two of the authors independently followed selection principles, and results from each screening step were always accomplished by consensus. Agreement strategies (kappa coefficient) were reported all along the process to assure reliability of the results. And finally, data extracted from the original studies was clearly delineated. Since relevant studies varied in quality and in methodology, one of the authors constructed a matrix of findings, which were furtherly revised by a second author to assure consistency of the information extracted. As limitations of the present SystRev, within the HMS it is considered that some strategies cannot be included due to lack of compliance, or the application of the same strategy among one study and another may have a different degree of compliance and interpretation. Although the strategies found are classified into three categories, it is not mandatory to follow this classification in future publications about PTB control, but we consider that the three grouped strategies found herein respond to MAP's control objectives. Publication bias was not controlled since the results of the control programs in endemic countries may be consigned in state government authorities and not as review material by a specialized public. Language bias was also considered, however, comprehensive literature searches followed by a careful assessment of study quality are required to assess the contribution of all relevant trials, independent of language of publication (Jüni et al., 2002). In addition, the databases selected for the primary search of citations, responded to the ease of access from the role of research authors, also being considered by the same as the most accurate for the search of content of health and animal sciences, according to previous experience. Finally, the databases used (MEDLINE, Embase) correspond to databases reported optimal as a minimum requirement to guarantee adequate and efficient coverage in SystRev on health-related topics (Bramer et al., 2017). In conclusion, the main PTB control strategies reported in dairy cattle are HMS, TCS, and VS. Within HMS, the preventive practices of removing the calves from its dam as soon as possible after birth and the minimal exposure of calves and heifers to adult cattle manure are the most used within the selected studies. HMS is used based on ELISA tests in animals > 2 years of age, culling different risk populations such as clinical animals, positive animals, off springs, and fecal shedders. The VS takes its importance to reduce fecal excretion of MAP and clinical presentation of PTB. The use of one or several combined strategies, considering the production and management practices, has shown to be successful in controlling the PTB in dairy cattle. Conflict of interestThe authors declare that there is no conflict of interest. Author contributionsAll authors contributed to the study conception and design. BT had the idea of the article. The literature searches and data analysis, as well as the critical revision of the manuscript was performed by all the authors. The first draft of the manuscript was written by BT and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Availability of data, code, and other materialsThe review protocol, the template for data collection forms and data extracted from included studies are available upon request to the corresponding author. ReferencesAlonso-Hearn, M., Molina, E., Geijo, M., Vázquez, P., Sevilla, I.A., Garrido, J.M. and Juste, R.A. 2012. Immunization of adult dairy cattle with a new heat-killed vaccine is associated with longer productive life prior to cows being sent to slaughter with suspected paratuberculosis. J. Dairy Sci. 95, 618–629. Aly, S.S., Gardner, I. A., Adaska, J.M. and Anderson, R.J. 2015. Off-site rearing of heifers reduces the risk of Mycobacterium avium ssp. paratuberculosis ELISA seroconversion and fecal shedding in a California dairy herd. J. Dairy Sci. 98, 1805–1814. Arango-Sabogal, J.C., Paré, J., Labrecque, O., Côté, G., Roy, J.P., Buczinski, S., Wellemans, V. and Fecteau, G. 2017. Incidence of fecal excretion of Mycobacterium avium subsp. paratuberculosis in dairy cows before and after the enrolment in the Québec voluntary program. Prev. Vet. Med. 148, 94–105. Bastida, F. and Juste, R.A. 2011. Paratuberculosis control: a review with a focus on vaccination. J. Immune Based. Ther. Vaccines. 9, 8; . Behr, M.A. and Collins, D.M. 2010. Paratuberculosis: organism, disease, control, 1st ed. Oxfordshire, UK: CABI, pp: 375. Behr, M.A., Stevenson, K. and Kapur, V. 2020. Paratuberculosis: organism, disease, control, 2nd ed. Oxfordshire, UK: CABI, pp: 426. Benedictus, A., Mitchell, R.M., Linde-Widmann, M., Sweeney, R., Fyock, T., Schukken, Y.H. and Whitlock, R.H. 2008. Transmission parameters of Mycobacterium avium subspecies paratuberculosis infections in a dairy herd going through a control program. Prev. Vet. Med. 83, 215–227. Bramer, W.M., Rethlefsen, M.L., Kleijnen, J. and Franco O.H. 2017. Optimal database combinations for literature searches in systematic reviews: a prospective exploratory study. Syst. Rev. 6, 245. Camanes, G., Joly, A., Fourichon, C., Ben Romdhane, R. and Ezanno, P. 2018. Control measures to prevent the increase of paratuberculosis prevalence in dairy cattle herds: an individual-based modelling approach. Vet. Res. 49(1), 60. Caldow, G., Crawshaw, M., Rusbridge, S. and Gunn, G. 2001. Johne's disease control programmes. Vet. Rec. 149, 192. Clarke, C.J. 1997. The pathology and pathogenesis of paratuberculosis in ruminants and other species. J. Comp. Pathol. 116, 217–261. Click, R.E. 2011. A 60-day probiotic protocol with Dietzia subsp. C79793-74 prevents development of Johne's disease parameters after in utero and/or neonatal MAP infection. Virulence 2, 337–347. Coad, M., Clifford, D.J., Vordermeier, H.M. and Whelan, A.O. 2013. The consequences of vaccination with the Johne's disease vaccine, Gudair, on diagnosis of bovine tuberculosis. Vet. Rec. 172, 266. Collins, M.T., Eggleston, V. and Manning, E.J. 2010. Successful control of Johne's disease in nine dairy herds: results of a six-year field trial. J. Dairy Sci. 93, 1638–1643. Correia-Gomes, C., Mendonça, D. and Niza-Ribeiro, J. 2010. Risk associations to milk ELISA result for paratuberculosis in dairy cows in Northern Portugal using a multilevel regression model. Rev. Méd. Vét. 161, 295–301. Donat, K. 2016. The Thuringian bovine paratuberculosis control programme – results and experiences Das Thüringer Programm zur Bekämpfung der Paratuber kulose in Rinderherden – Ergebnisse und Erfahrungen. BMTW. 129. Donat, K., Schmidt, M., Köhler, H. and Sauter-Louis, C. 2016. Management of the calving pen is a crucial factor for paratuberculosis control in large dairy herds. J. Dairy Sci. 99(5), 3744–3752. Doré, E., Paré, J., Côté, G., Buczinski, S., Labrecque, O., Roy, J.P. and Fecteau, G. 2012. Risk factors associated with transmission of Mycobacterium avium subsp. paratuberculosis to calves within dairy herd: a systematic review. J. Vet. Inter. Med. 26, 32–45. Eisenberg, S., Nielen, M., Hoeboer, J., Bouman, M., Heederik, D. and Koets, A. 2011. Mycobacterium avium subspecies paratuberculosis in bioaerosols after depopulation and cleaning of two cattle barns. Vet. Rec. 168, 587. Eltholth, M.M., Marsh, V.R., Van Winden, S. and Guitian, F.J. 2009. Contamination of food products with Mycobacterium avium paratuberculosis: a systematic review. J. Appl. Microbiol. 107, 1061–1071. Espejo, L.A., Godden, S., Hartmann, W. L. and Wells, S.J. 2012. Reduction in incidence of Johne's disease associated with implementation of a disease control program in Minnesota demonstration herds. J. Dairy Sci. 95, 4141–4152. Feller, M., Huwiler, K., Stephan, R., Altpeter, E., Shang, A., Furrer, H., Pfyffer, G. E., Jemmi, T., Baumgartner, A. and Egger, M. 2007. Mycobacterium avium subspecies paratuberculosis and Crohn's disease: a systematic review and meta-analysis. Lancet Infect. Dis. 7, 607–613. Ferrouillet, C., Wells, S.J., Hartmann, W.L., Godden, S.M. and Carrier, J. 2009. Decrease of Johne's disease prevalence and incidence in six Minnesota, USA, dairy cattle herds on a long-term management program. Prev. Vet. Med. 88, 128–137. Fox, N.J., Caldow, G.L., Liebeschuetz, H., Stevenson, K. and Hutchings, M.R. 2018. Counterintuitive increase in observed Mycobacterium avium subspecies paratuberculosis prevalence in sympatric rabbits following the introduction of paratuberculosis control measures in cattle. Vet. Rec. 182, 634. Garry, F. 2011. Control of paratuberculosis in dairy herds. Vet. Clin. North Am. Food Anim. Pract. 27, 599–607. Geraghty, T., Graham, D.A., Mullowney, P. and More, S.J. 2014. A review of bovine Johne's disease control activities in 6 endemically infected countries. Prev. Vet. Med. 116, 1–11. Gilardoni, L.R., Paolicchi, F.A. and Mundo, S.L. 2012. Bovine paratuberculosis: a review of the advantages and disadvantages of different diagnostic tests. Rev. Argent. Microbiol. 44, 201–215. Gonda, M.G., Chang, Y.M., Shook, G.E., Collins, M.T. and Kirkpatrick, B.W. 2007. Effect of Mycobacterium paratuberculosis infection on production, reproduction, and health traits in US Holsteins. Prev. Vet. Med. 80, 103–119. Hutchinson, L.J. 1996. Economic impact of paratuberculosis. Vet. Clin. North Am. Food Anim. Pract. 12, 373–381. Johnson-Ifearulundu, Y.J. and Kaneene, J.B. 1998. Management-related risk factors for M. paratuberculosis infection in Michigan, USA, dairy herds. Prev. Vet. Med. 37, 41–54. Johnson-Ifearulundu, Y. and Kaneene, J.B. 1999. Distribution and environmental risk factors for paratuberculosis in dairy cattle herds in Michigan. Am. J. Vet. Res. 60, 589–596. Jubb, T. and Galvin, J. 2000. Herd testing to control bovine Johne's disease. Vet. Microbiol. 77(3–4), 423–428. Jubb, T.F. and Galvin, J.W. 2004. Effect of a test and control program for bovine Johne's disease in Victorian dairy herds 1992–2002. Aust. Vet. J. 82, 228–232. Jüni, P., Holenstein, F., Sterne, J., Bartlett, C. and Egger, M. 2002. Direction and impact of language bias in meta-analyses of controlled trials: empirical study. Int. J. Epidemiol. 31, 115–123. Juste, R.A., Alonso-Hearn, M., Molina, E., Geijo, M., Vazquez, P., Sevilla, I.A. and Garrido, J.M. 2009. Significant reduction in bacterial shedding and improvement in milk production in dairy farms after the use of a new inactivated paratuberculosis vaccine in a field trial. BMC Res. Notes 2, 233. Juste, R.A., Geijo, M.V., Elguezabal, N., Sevilla, I.A., Alonso-Hearn, M. and Garrido, J.M. 2021. Paratuberculosis vaccination specific and non-specific effects on cattle lifespan. Vaccine 39(11), 1631–1641. Kalis, C.H., Hesselink, J.W., Barkema, H.W. and Collins, M.T. 2001. Use of long-term vaccination with a killed vaccine to prevent fecal shedding of Mycobacterium avium subsp paratuberculosis in dairy herds. Am. J. Vet. Res. 62, 270–274. Kennedy, D.J. and Benedictus, G. 2001. Control of Mycobacterium avium subsp. paratuberculosis infection in agricultural species. Rev. Sci. Tech. (OIE). 20, 151–179. Klopfstein, M., Leyer, A., Berchtold, B., Torgerson, P. R. and Meylan, M. 2021. Limitations in the implementation of control measures for bovine paratuberculosis in infected Swiss dairy and beef herds. PLoS One 16(2), e0245836. Konboon, M., Bani-Yaghoub, M., Pithua, P.O., Rhee, N. and Aly, S.S. 2018. A nested compartmental model to assess the efficacy of paratuberculosis control measures on U.S. dairy farms. PLoS One 13, e0203190. Körmendy, B. 1994. The effect of vaccination on the prevalence of paratuberculosis in large dairy herds. Vet. Microbiol. 41, 117–125. Künzler, R., Torgerson, P., Keller, S., Wittenbrink, M., Stephan, R., Knubben-Schweizer, G., Berchtold, B. and Meylan, M. 2014. Observed management practices in relation to the risk of infection with paratuberculosis and to the spread of Mycobacterium avium subsp. paratuberculosis in Swiss dairy and beef herds. BMC Vet. Res. 10, 132. Lee, A., Griffiths, T.A., Parab, R.S., King, R. K., Dubinsky, M.C., Urbanski, S.J., Wrobel, I. and Rioux, K.P. 2011. Association of Mycobacterium avium subspecies paratuberculosis with Crohn Disease in pediatric patients. JPGN. 52, 170–174. Lombard, J.E., Garry, F.B., McCluskey, B.J. and Wagner, B.A. 2005. Risk of removal and effects on milk production associated with paratuberculosis status in dairy cows. JAVMA. 227, 1975–1981. Manning, E.J.B. and Collins, M.T. 2010. Epidemiology of Paratuberculosis. In Paratuberculosis: organism, disease, control, Eds., Behr, M.A. and Collins, D.M. Oxfordshire, UK: CABI, pp: 22–29. Marcé, C., Ezanno, P., Weber, M. F., Seegers, H., Pfeiffer, D.U. and Fourichon, C. 2010. Invited review: modeling within-herd transmission of Mycobacterium avium subspecies paratuberculosis in dairy cattle: a review. J. Dairy Sci. 93, 4455–4470. Nielsen, S.S. and Toft, N. 2008. Ante mortem diagnosis of paratuberculosis: a review of accuracies of ELISA, interferon-gamma assay, and faecal culture techniques. Vet. Microbiol. 129, 217–235. Nielsen, S.S. and Toft, N. 2011. Effect of management practices on paratuberculosis prevalence in Danish dairy herds. J. Dairy Sci. 94, 1849–1857. Obasanjo, I.O., Gröhn, Y.T. and Mohammed, H.O. 1997. Farm factors associated with the presence of Mycobacterium paratuberculosis infection in dairy herds on the New York State Paratuberculosis Control Program. Prev. Vet. Med. 32, 243–251. Ott, S.L., Wells, S.J. and Wagner, B.A. 1999. Herd-level economic losses associated with Johne's disease on US dairy operations. Prev. Vet. Med. 40, 179–192. Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Shamseer, L., Tetzlaff, J. M., Akl, E. A., Brennan, S. E., Chou, R., Glanville, J., Grimshaw, J. M., Hróbjartsson, A., Lalu, M. M., Li, T., Loder, E. W., Mayo-Wilson, E., McDonald, S., McGuinness, L. A. and Moher, D. 2021. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical research ed.). 372, n71. Palmer, M.V. and Waters, W.R. 2011. Bovine tuberculosis and the establishment of an eradication program in the United States: role of veterinarians. Vet. Med. Int. 2011, 816345. Patton, E.A. 2011. Paratuberculosis vaccination. Vet. Clin. North Am. Food Anim. Pract. 27, 573–580. Pieper, L., DeVries, T.J., Sorge, U.S., Godkin, A., Hand, K.J., Perkins, N.R., Imada, J. and Kelton, D.F. 2015. Variability in risk assessment and management plan (RAMP) scores completed as part of the Ontario Johne's Education and Management Assistance Program (2010–2013). J. Dairy Sci. 98, 2419–2426. Pillars, R.B., Grooms, D.L., Gardiner, J.C. and Kaneene, J.B. 2011. Association between risk-assessment scores and individual-cow Johne's disease-test status over time on seven Michigan, USA dairy herds. Prev. Vet. Med. 98, 10–18. Pillars, R.B., Grooms, D.L., Wolf, C.A. and Kaneene, J.B. 2009. Economic evaluation of Johne's disease control programs implemented on six Michigan dairy farms. Prev. Vet. Med. 90, 223–232. Pithua, P., Espejo, L.A., Godden, S.M. and Wells, S.J. 2013. Is an individual calving pen better than a group calving pen for preventing transmission of Mycobacterium avium subsp paratuberculosis in calves? Results from a field trial. Res. Vet. Sci. 95, 398–404. Pithua, P., Godden, S.M., Wells, S.J. and Oakes, M.J. 2009. Efficacy of feeding plasma-derived commercial colostrum replacer for the prevention of transmission of Mycobacterium avium subsp paratuberculosis in Holstein calves. JAVMA. 234, 1167–1176. Puerto-Parada, M., Arango-Sabogal, J.C., Paré, J., Doré, E., Côté, G., Wellemans, V., Buczinski, S., Roy, J.P., Labrecque, O. and Fecteau, G. 2018. Risk factors associated with Mycobacterium avium subsp. paratuberculosis herd status in Québec dairy herds. Prev. Vet. Med. 152, 74–80. Reddacliff, L., Eppleston, J., Windsor, P., Whittington, R. and Jones, S. 2006. Efficacy of a killed vaccine for the control of paratuberculosis in Australian sheep flocks. Vet. Microbiol. 115, 77–90. Richardson, E. and More, S. 2009. Direct and indirect effects of Johne's disease on farm and animal productivity in an Irish dairy herd. Ir. Vet. J. 62, 526–532. Ridge, S.E., Baker, I.M. and Hannah, M. 2005. Effect of compliance with recommended calf-rearing practices on control of bovine Johne's disease. Aust. Vet. J. 83, 85–90. Roche, S.M., Kelton, D.F., Meehan, M., Von Massow, M. and Jones-Bitton, A. 2019. Exploring dairy producer and veterinarian perceptions of barriers and motivators to adopting on-farm management practices for Johne's disease control in Ontario, Canada. J. Dairy Sci. 102, 4476–4488. Sechi, L.A. and Dow, C.T. 2015. Mycobacterium avium ss. paratuberculosis zoonosis - The hundred year war - beyond Crohn's disease. Front Immunol. 6, 96. Serrano, M., Elguezabal, N., Sevilla, I.A., Geijo, M.V., Molina, E., Arrazuria, R., Urkitza, A., Jones, G.J., Vordermeier, M., Garrido, J.M. and Juste, R.A. 2017. tuberculosis detection in paratuberculosis vaccinated calves: New alternatives against interference. PLoS One 12, e0169735. Smith, R.L., Strawderman, R.L., Schukken, Y.H., Wells, S.J., Pradhan, A.K., Espejo, L.A., Whitlock, R.H., Van Kessel, J.S., Smith, J.M., Wolfgang, D.R. and Gröhn, Y.T. 2010. Effect of Johne's disease status on reproduction and culling in dairy cattle. J. Dairy Sci. 93, 3513–3524. Stabel, J.R. 2008. Pasteurization of colostrum reduces the incidence of paratuberculosis in neonatal dairy calves. J. Dairy Sci. 91, 3600–3606. Sweeney, R.W., Collins, M.T., Koets, A.P., McGuirk, S.M. and Roussel, A.J. 2012. Paratuberculosis (Johne's disease) in cattle and other susceptible species. J. Vet. Intern Med. 26, 1239–1250. Wells, S.J. and Wagner, B.A. 2000. Herd-level risk factors for infection with Mycobacterium paratuberculosis in US dairies and association between familiarity of the herd manager with the disease or prior diagnosis of the disease in that herd and use of preventive measures. JAVMA. 216, 1450–1457. Wentink, G.H., Bongers, J.H., Zeeuwen, A.A. and Jaartsveld, F.H. 1994. Incidence of paratuberculosis after vaccination against M. paratuberculosis in two infected dairy herds. Zentralblatt fur Veterinarmedizin. Reihe B. J. Vet. Med. (Series B). 41, 517–522. Whittington, R., Donat, K., Weber, M. F., Kelton, D., Nielsen, S. S., Eisenberg, S., Arrigoni, N., Juste, R., Sáez, J. L., Dhand, N., Santi, A., Michel, A., Barkema, H., Kralik, P., Kostoulas, P., Citer, L., Griffin, F., Barwell, R., Moreira, M., Slana, I. and de Waard, J.H. 2019. Control of paratuberculosis: who, why and how. A review of 48 countries. BMC Vet Res. 15, 198. Wilesmith, J.W. 1982. Johne's disease: a retrospective study of vaccinated herds in Great Britain. Br. Vet. J. 138(4), 321–331. Yamasaki, E.M., Tokarnia, C.H., Galvão, A., Gomes, M.J.P, Chies, J.A.B, Veit, T.D., Aragão, A.P. and Brito, M.F. 2010. Aspectos clínico-patológicos e controle da paratuberculose em rebanho bovino leiteiro. Pesq. Vet. Bras. 30, 921–932. | ||
| How to Cite this Article |
| Pubmed Style Tuberquia-lópez BC, Correa-valencia NMDP, Hernández JM, Fernández-silva JA, Ramírez-vásquez NF. Paratuberculosis control strategies in dairy cattle: A systematic review. Open Vet. J.. 2022; 12(4): 525-539. doi:10.5455/OVJ.2022.v12.i4.16 Web Style Tuberquia-lópez BC, Correa-valencia NMDP, Hernández JM, Fernández-silva JA, Ramírez-vásquez NF. Paratuberculosis control strategies in dairy cattle: A systematic review. https://www.openveterinaryjournal.com/?mno=95188 [Access: December 02, 2025]. doi:10.5455/OVJ.2022.v12.i4.16 AMA (American Medical Association) Style Tuberquia-lópez BC, Correa-valencia NMDP, Hernández JM, Fernández-silva JA, Ramírez-vásquez NF. Paratuberculosis control strategies in dairy cattle: A systematic review. Open Vet. J.. 2022; 12(4): 525-539. doi:10.5455/OVJ.2022.v12.i4.16 Vancouver/ICMJE Style Tuberquia-lópez BC, Correa-valencia NMDP, Hernández JM, Fernández-silva JA, Ramírez-vásquez NF. Paratuberculosis control strategies in dairy cattle: A systematic review. Open Vet. J.. (2022), [cited December 02, 2025]; 12(4): 525-539. doi:10.5455/OVJ.2022.v12.i4.16 Harvard Style Tuberquia-lópez, B. C., Correa-valencia, . N. M. D. P., Hernández, . J. M., Fernández-silva, . J. A. & Ramírez-vásquez, . N. F. (2022) Paratuberculosis control strategies in dairy cattle: A systematic review. Open Vet. J., 12 (4), 525-539. doi:10.5455/OVJ.2022.v12.i4.16 Turabian Style Tuberquia-lópez, Brahian Camilo, Nathalia María Del Pilar Correa-valencia, José Miguel Hernández, Jorge Arturo Fernández-silva, and Nicolas Fernando Ramírez-vásquez. 2022. Paratuberculosis control strategies in dairy cattle: A systematic review. Open Veterinary Journal, 12 (4), 525-539. doi:10.5455/OVJ.2022.v12.i4.16 Chicago Style Tuberquia-lópez, Brahian Camilo, Nathalia María Del Pilar Correa-valencia, José Miguel Hernández, Jorge Arturo Fernández-silva, and Nicolas Fernando Ramírez-vásquez. "Paratuberculosis control strategies in dairy cattle: A systematic review." Open Veterinary Journal 12 (2022), 525-539. doi:10.5455/OVJ.2022.v12.i4.16 MLA (The Modern Language Association) Style Tuberquia-lópez, Brahian Camilo, Nathalia María Del Pilar Correa-valencia, José Miguel Hernández, Jorge Arturo Fernández-silva, and Nicolas Fernando Ramírez-vásquez. "Paratuberculosis control strategies in dairy cattle: A systematic review." Open Veterinary Journal 12.4 (2022), 525-539. Print. doi:10.5455/OVJ.2022.v12.i4.16 APA (American Psychological Association) Style Tuberquia-lópez, B. C., Correa-valencia, . N. M. D. P., Hernández, . J. M., Fernández-silva, . J. A. & Ramírez-vásquez, . N. F. (2022) Paratuberculosis control strategies in dairy cattle: A systematic review. Open Veterinary Journal, 12 (4), 525-539. doi:10.5455/OVJ.2022.v12.i4.16 |