| Original Article | ||
Open Vet. J.. 2022; 12(4): 445-450 Open Veterinary Journal, (2022), Vol. 12(4): 445–450 Original Research Felbamate as an oral add-on therapy in six dogs with presumptive idiopathic epilepsy and generalized seizures resistant to drug therapyCurtis Wells Dewey1*, Mark Rishniw2 and Kasie Sakovitch11Elemental Pet Vets, PLLC, Freeville, New York, USA 2Department of Clinical Sciences, College of Veterinary Medicine, Cornell University, Ithaca, New York, USA *Corresponding Author: Curtis W. Dewey. Elemental Pet Vets, PLLC, 1610 Dryden Road, Freeville, NY 13068, USA. Email: elementalpetvets [at] outlook.com Submitted: 08/03/2022 Accepted: 13/06/2022 Published: 11/07/2022 © 2022 Open Veterinary Journal
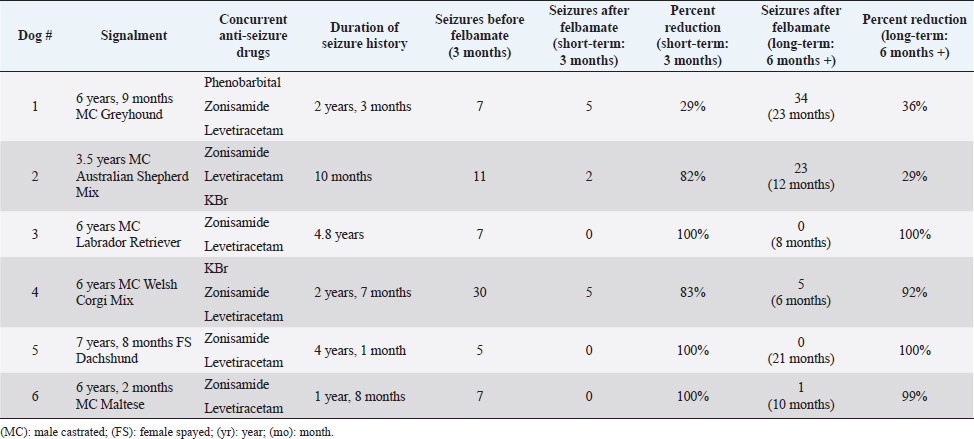
AbstractBackground: Idiopathic or genetic epilepsy commonly affects dogs; affected dogs are often refractory to anti-seizure drug therapy. Felbamate is an anti-seizure drug with established pharmacokinetic and safety data for dogs, but little published evidence of efficacy for managing generalized seizures in this species. Aim: The purpose of this retrospective case series was to evaluate the clinical efficacy and tolerability of oral felbamate in six presumptive epileptic dogs experiencing generalized seizures. Methods: Medical records from six dogs with presumptive idiopathic/genetic epilepsy manifesting as generalized seizure activity, for which oral felbamate was used as an add-on treatment, were reviewed. The number of seizures recorded for the 3-month period immediately before instituting felbamate was recorded for each dog. Short-term (3 months) and long-term (6 months or greater) seizure frequency post-felbamate therapy was recorded for each dog and compared with baseline. Results: Overall, dogs experienced a reduction (82%) in seizures after adding felbamate in the short term, with 5/6 dogs (83%) classified as responders (50% or greater reduction in seizures) and 3/6 dogs (50%) attaining seizure-free status. Mean and median long-term follow-up times were 13 and 11 months, respectively (range: 6 to 23 months). Four of the 6 dogs (67%) remained drug responders at final follow-up, with an average seizure reduction of 98%, 2 of which remained seizure-free at 8 and 21 months. Two dogs (33%) experienced increased seizure activity during long-term follow-up (12 and 23 months) and were considered non-responders. The non-responder dogs had an average long-term seizure reduction of 33%. No dog experienced any obvious adverse effects associated with felbamate administration. However, one dog not included in the analysis because of insufficient (<3 month) post-felbamate follow-up, was weaned off felbamate because of suspected hepatotoxicity. Conclusion: Our small case series suggests that oral felbamate might show promise as an add-on drug for epileptic dogs experiencing generalized seizures resistant to drug therapy. These results warrant a more controlled, prospective investigation into felbamate as a therapeutic agent for canine epilepsy. Keywords: Canine, Seizures, Brain, Felbamate. IntroductionIdiopathic epilepsy (IE), also termed genetic epilepsy, is a common neurologic disease affecting dogs; approximately 25% to 30% of these patients are refractory to standard antiseizure drug therapy (Munana, 2013; Volk, 2014; Thomas and Dewey, 2016). The need for effective antiseizure drugs for dogs with minimal adverse effects remains, despite the addition of several new therapeutic options over the years. Several drugs used for seizure control in epileptic dogs cause obvious adverse effects (e.g., sedation, polydipsia/polyuria, and polyphagia/weight gain). Other antiseizure drugs that are typically used as add-on therapies have less tendency for adverse effects but might have less efficacy for reducing seizure frequency as first-line drugs, when compared to phenobarbital or bromide (Munana, 2013; Volk, 2014; Packer and Volk, 2015; Thomas and Dewey, 2016). Felbamate (2-phenyl-1,3-propanediol dicarbamate) is an antiseizure drug approved for human use in the United States in 1993. Felbamate’s mechanisms of action appear diverse: it inhibits excitatory N-methyl-D-aspartate (NMDA) receptor activity and potentiates inhibitory gamma aminobutyric acid (GABA) receptor activity (Platt, 2014; Leppik, 1995; Leppik and Wolf, 1995; Bourgeois, 1997). Felbamate also has neuroprotective effects against neuronal hypoxia in animal models (Wasterlain et al., 1992; Chronopoulos et al., 1993; Wallis and Panizzon, 1993). An attractive feature of felbamate is its lack of sedation (Bourgeois, 1997; Zupanc et al., 2010). Despite its apparent efficacy and lack of adverse effects, reports of fatal aplastic anemia and hepatoxicity in a small percentage of people receiving the drug appeared during the first year of its release; these uncommon yet serious complications prompted a warning from regulatory agencies regarding felbamate use in humans (Leppik, 1995; Leppik and Wolf, 1995; Bourgeois, 1997; Thakkar et al., 2015). Although the pharmacokinetics and tolerability of oral felbamate in normal dogs are well established (Adusumalli et al., 1991, 1992; Yang et al., 1992; McGee et al., 1998), literature regarding use in epileptic dogs is limited to one abstract and one published case series (Ruehlmann et al., 2001). Dayrell Hart et al. (1996), in the abstract, 16 dogs with refractory seizures (unspecified cause) had felbamate added to phenobarbital and potassium bromide therapy; 12 dogs had what the authors claimed was improved seizure control, however, the authors did not specify seizure type (focal vs. generalized) or extent of seizure reduction. In this report, four dogs developed liver disease after initiation of felbamate. In a case series of six dogs with focal seizure activity, the addition of felbamate reduced seizure frequency in all dogs and the authors did not report any serious adverse effects. Two dogs in that case series developed reversible blood dyscrasias (thrombocytopenia, leukopenia) and one dog developed keratoconjunctivitis sicca (Ruehlmann et al., 2001). To the authors’ knowledge, there are no published studies regarding the use of felbamate in dogs with idiopathic/genetic epilepsy experiencing generalized seizure activity. One of the authors (CD) of this report has considerable anecdotal experience using felbamate in dogs with generalized seizures. Therefore, we sought to evaluate the efficacy and tolerability of oral felbamate as an add-on drug in dogs with presumptive idiopathic/genetic epilepsy experiencing generalized seizures. Materials and MethodsWe searched medical records of Elemental Pet Vets’ database for dogs diagnosed with presumptive IE and generalized seizures treated with oral felbamate as an add-on drug between 2019 and 2021. All dogs were examined and treated by a board-certified neurologist (CD). We based a diagnosis of presumptive IE on each dog satisfying the following criteria, similar to previous studies (Dewey et al., 2004, 2009; Kiviranta et al., 2013): 1) onset of generalized seizure activity between 1 and 5 years of age, 2) intermittent seizures for more than 6 months, 3) normal neurologic examination during the inter-ictal period, and 4) results of hematologic analysis (i.e., CBC and serum biochemical analysis) within the respective reference ranges (except for those abnormalities attributable to anti-seizure medications). Dogs whose seizure history began after 5 years of age were considered if they met the other stated criteria and had normal magnetic resonance imaging of the brain. Dogs were eligible for inclusion if they experienced an average of two or more seizures per month, despite receiving appropriate doses of at least two antiseizure drugs. Seizure frequency data were recorded for each dog based on information obtained from medical records, owners’ seizure logs, and/or owner questionnaires. Each dog required a minimum seizure history of 6 months before felbamate initiation. In cases of cluster seizures, in which the number of seizures was not clearly documented, the cluster was counted as one seizure event. Seizure frequency was tabulated for the 3-month pre-felbamate treatment period (short-term follow-up) and a long-term follow-up period of at least 6 months. Cases were excluded if they required an increase in anti-seizure drug doses or addition of new anti-seizure drugs during the 3-month post-felbamate evaluation period. Adverse effects attributable to felbamate administration were recorded, including any changes in bloodwork. Pre-felbamate seizure numbers were estimated based on either examination of seizure logs maintained by the clients, or on communication with the client and examination of medical records (in one dog). Individual patient success was defined as a minimum of 50% seizure frequency reduction (i.e., responder) when comparing the felbamate treatment period (short-and long-term) to the preceding 3-month time. For those dogs that had dose escalations of felbamate shortly after felbamate institution, the post-felbamate evaluation period was restricted to the 3 months following the final dose adjustment. Total seizure numbers were compared between the pre-and short-term post-felbamate treatment periods. Since long-term follow-up periods were variable among patients, average monthly seizures were compared between pre-felbamate and long-term follow-up time periods (vs. total seizure numbers). Given the small number of dogs, we omitted statistical analyses and restricted our results to descriptive analyses. ResultsWe identified 12 presumptively epileptic dogs that had received oral felbamate as an add-on drug; six met the study criteria (Table 1). Of the six dogs who did not meet the study criteria, two required an additional drug within 3 months of initiating felbamate treatment, one dog experienced only focal seizure activity, and one dog experienced inappetence, weight loss, and elevated liver enzymes (attributed to felbamate) within the 3-month surveillance period. Felbamate was discontinued in this dog prior to reaching a 3-month post-felbamate evaluation period. Two additional dogs were excluded due to insufficient follow-up time after final felbamate dose adjustment. All six dogs had experienced cluster seizure activity during the pre-felbamate evaluation period. Clients maintained accurate seizure logs for 5/6 dogs; the remaining dog had five cluster episodes (number of seizures per cluster not specified) in the pre-felbamate evaluation period and was seizure-free during the post-felbamate evaluation period. Seizure history for the six dogs ranged from 10 months to 4.8 years (median=2.4 years). The dogs were receiving two to three anti-seizure drugs at the time of felbamate initiation (Table 1). Felbamate doses ranged from 15.2 to 21.3 mg/kg body weight (median=18.2 mg/kg), q 8 (2 dogs) to 12 (4 dogs) hours. Two dogs required dose escalation from their initial felbamate dose before achieving seizure control. All dogs showed a reduction in seizures (median=91.5%) after treatment with felbamate in the short-term. Five dogs (83%) were designated responders, experiencing more than 50% seizure reduction during the 3-month post-felbamate period. Three dogs (50%) became seizure free during the 3-month post-felbamate period. Complete blood counts and serum biochemical analyses, procured between 2 and 14 months following felbamate initiation in all dogs, showed mild elevations of alanine aminotransferase (ALT: 158 U/l; normal range, 10–125 U/l) and alkaline phosphatase (ALP: 546 U/l; normal range, 23–212 U/l) in only one dog. No obvious adverse effects attributable to felbamate were reported in any of these dogs. Dogs were followed beyond the initial 3-month period, for a median of 11 months (range: 6 to 23 months). Two of the initially seizure-free dogs remained seizure-free for the duration of long-term follow-up (8 and 21 months). The third seizure-free dog remained so for 7 months, experienced a single seizure, and then remained seizure-free for another 3 months after increasing felbamate from q 12 to q 8 hours (total of 10 months follow-up). One dog that experienced an 83% reduction in seizures in the short-term period experienced no further seizures in the subsequent 3 months (total 6-month follow-up). Overall, the four responder dogs had an average of 98% seizure reduction at long-term follow-up (mean, 11 months; median, 9 months). One dog (Dog #1) that had experienced a 29% short-term seizure reduction had 29 more seizures in the ensuing 20 months and was considered a drug failure (non-responder). This patient was also receiving an additional anti-seizure drug (topiramate) for the year preceding the final follow-up. One dog (Dog #2) that had experienced an 82% short-term seizure reduction experienced an increase in seizures over the ensuing 9 months of follow-up, experiencing an additional 21 seizures during this time. This patient was also considered a non-responder, with a long-term seizure reduction of 29%. Table 1. Summary of pre-and post-felbamate seizures in six dogs.
DiscussionThe results of our retrospective case series suggest that felbamate might be an effective add-on drug for dogs with presumptive IE experiencing generalized seizures that are inadequately controlled with first-line anti-seizure drugs. In addition to an overall seizure reduction in the short-term post-felbamate period, we considered 5/6 dogs (83%) responders (≥50% seizure reduction); three of these (50%) became seizure-free. Furthermore, 4/6 dogs in this case series experienced a long-term reduction in seizure frequency after instituting felbamate. There were no apparent adverse effects associated with felbamate administration in these dogs. Although one dog had evidence of mildly elevated liver enzymes after initiating felbamate therapy, we were not able to ascertain if these bloodwork changes were attributable to felbamate or other anti-seizure drugs administered concurrently. The liver enzyme elevations were not considered severe enough to discontinue felbamate therapy. Despite this, one dog was excluded from the study due to suspected felbamate-induced hepatotoxicity. This dog has weaned off felbamate before the end of the 3-month post-felbamate evaluation period. The patient’s liver enzymes improved shortly after felbamate discontinuation. We did not conduct a prospective randomized controlled trial. The “gold standard” for conducting clinical trials to investigate the efficacy of an anti-seizure drug is a double-blinded, placebo-controlled study design (Charalambous et al., 2014). There are numerous impediments to adhering to this standard when investigating anti-seizure medications, including costs involved, difficulty in recruiting cases (owners of refractory epileptic dogs are reticent to agree to a placebo administration period), time commitment, and ethical considerations (Packer et al., 2015). Indeed, evidence from human epilepsy drug trials suggests an increased mortality risk in the placebo group (Hesdorffer et al., 2011; Ryvlin et al., 2011). Uncontrolled, open clinical investigations, although inherently more flawed than the “gold standard”, can provide useful information that may help clinicians decide whether further investigation (i.e., controlled prospective study) is warranted (Packer et al., 2015; Dewey et al., 2020). Because of the multiple limitations of a retrospective case series, the results of this investigation should be considered with caution. In addition to a small number of cases, owners were not blinded to the therapy being administered and there was no control group. Also, two dogs that were excluded from this series had insufficient post-felbamate evaluation time (<3 months) for inclusion. Both of these dogs required additional anti-seizure medications soon after felbamate institution, precluding them from the analysis. Although these two dogs did not fit our criteria for inclusion, they should be considered drug failures from a practical clinical perspective. However, even with the inclusion of these two dogs, 50% (4/8) of the dogs receiving felbamate as an add-on drug would have been considered responders at the end of the long-term follow-up period. Data accumulated retrospectively are more likely to be inaccurate compared with prospective investigations, particularly data concerning seizure numbers. In our case series, one dog did not have an accurate pre-felbamate seizure log and the medical records indicated five episodes of cluster seizures in the pre-felbamate time period; the owner had maintained a seizure log and discarded it after the patient had been seizure-free for over a year. Since the number of seizures per cluster was not mentioned in the record, we tallied five as the pre-felbamate seizure number. The accuracy of the pre-felbamate seizure numbers is suspect in this patient but it is important to note that this dog experienced cluster seizures and became seizure-free after felbamate institution. Despite the limitations of our report, we feel our results are consistent with prior reports of efficacy and tolerability when felbamate is used in a clinical setting. Adverse effects attributable to the clinical use of felbamate in dogs are not well-documented but include hepatotoxicity (four dogs), reversible blood dyscrasias (two dogs), and possibly keratoconjunctivitis sicca (one dog) (Dayrell Hart et al., 1996; Ruehlmann et al., 2001). None of the dogs in our case series experienced obvious adverse effects associated with felbamate administration. However, we had to discontinue felbamate administration in one of the four dogs excluded from analysis (due to <3-month post-felbamate follow-up) due to suspected hepatotoxicity. All dogs in the abstract describing felbamate administration, including the four that developed hepatotoxicity, were also receiving phenobarbital (Dayrell Hart et al., 1996). Felbamate is known to increase phenobarbital concentrations in people when the two drugs are given concurrently and this same phenomenon is likely to occur in dogs (Gidal and Zupanc, 1994; Reidenberg et al., 1995; Ruehlmann et al., 2001; Platt, 2014). The combination of phenobarbital and felbamate may pose a higher risk for hepatotoxicity compared to other drug combinations with felbamate. In our case series, only one dog was receiving concurrent phenobarbital. In the case series of six dogs with focal seizure activity treated with felbamate, the adverse effects noted in three dogs developed between 3 and 13 months after starting felbamate treatment (Ruehlmann et al., 2001). The dose range of felbamate used for the dogs in our case series was 15 to 20 mg/kg body weight, every 8 to 12 hours. Felbamate undergoes extensive hepatic metabolism in dogs with an elimination half-life between 4 and 6 hours (Adusumalli et al., 1991, 1992; Platt, 2014). Despite the short elimination half-life of felbamate, the authors have anecdotally had dogs respond well to q 12-hour dosing. Accordingly, we often start dogs on a q 12-hour felbamate dosing schedule and increase it to q 8-hour dosing as needed. Although the literature on the topic of dose recommendations for felbamate in dogs is sparse, q 8-hour dosing is more consistently suggested (Ruehlmann et al., 2001; Platt, 2014). Our results are consistent with that suggested dosing interval, as two of the six dogs were increased from q 12- to q 8-hour dosing, and one of the three dogs that remained on q 12-hour dosing was considered a non-responder. Despite being approved for clinical use in 1993, felbamate did not become available as a generic drug in the United States until 2011 (Thakkar et al., 2015). Based on one author’s (CD) experience, felbamate was often prohibitively expensive for owners of epileptic dogs before the introduction of the generic option. Though not a new drug, felbamate may represent a more recently available add-on therapy for the treatment of generalized seizures in dogs. In summary, the results of our retrospective case series suggest that felbamate could offer an effective add-on drug option to treat generalized seizures in dogs with presumptive idiopathic/genetic epilepsy. Though limited, our findings warrant further controlled, prospective evaluation of felbamate as an antiseizure drug for dogs. ReferencesAdusumalli, V.E., Yang, J.T., Wong, K.K., Kucharczyk M. and Sofia, R.D. 1991. Felbamate pharmacokinetics in the rat, rabbit, and dog. Drug Metab. Dispos. 19, 1116–1125. Adusumalli, V.E., Gilchrist, J.R., Wichman, J.K, Kucharczyk, N. and Sofia, R.D. 1992. Pharmacokinetics of felbamate in pediatric and adult beagle dogs. Epilepsia. 33, 955–960. Bourgeois, B.F. 1997. Felbamate. Semin. Pediatr. Neurol. 4, 3–8. Charalambous, M., Brodbelt, D. and Volk, H. 2014. Treatment in canine epilepsy-a systematic review. BMC Vet. Res. 10, 257–281. Chronopoulos, A., Stafstrom, C., Thurber, S., Hyde, P., Mikati, M. and Holmes, G.L. 1993. Neuroprotective effect of felbamate after kainic acid-induced status epilepticus. Epilepsia. 34, 359–366. Dayrell Hart, B., Tiches, D., Vite, C. and Steinberg, S.A. 1996. Efficacy and safety of felbamate as an anticonvulsant in dogs with refractory seizures. In Research abstract program of the 14th annual ACVIM forum. San Antonio, TX. Dewey, C.W., Guiliano, R., Boothe, D.M., Berg, J.M., Joseph, R.J. and Budsberg, S.C. 2004. Zonisamide therapy for refractory idiopathic epilepsy in dogs. J. Am. Anim. Hosp. Assoc. 40, 285–291. Dewey, C.W., Cerda-Gonzalez, S., Levine, J.M., Badgley, B.L., Ducote’, J.M., Silver, G.M., Cooper, J.C., Packer, R.A. and Lavely, J.A. 2009. Pregabalin as an adjunct to phenobarbital, potassium bromide, or a combination of phenobarbital and potassium bromide for treatment of dogs with suspected idiopathic epilepsy. J. Am. Vet. Med. Assoc. 235, 1442–1449. Dewey, C.W., Gridley, A. and Fletcher, A. 2020. Evaluation of the Chinese herbal formula Di Tan Tang as an oral add-on therapy for dogs with presumptive refractory idiopathic epilepsy: an open-label investigation in 8 dogs. Am. J. Trad. Chin. Vet. Med. 14, 1–6. Gidal, B.E. and Zupanc, M.L. 1994. Potential pharmacokinetic interaction between felbamate and phenobarbital. Ann. Pharmacother. 28, 455–458. Hesdorffer D., Tomson, T., Benn, E., Sander, J.W., Nilsson, L., Langan, Y., Walczak, T.S., Beghi, E., Brodie, M.J. and Hauser, A. 2011. Combined analysis of risk factors for SUDEP. Epilepsia. 52, 1150–1159. Kiviranta, A.M., Laitinen-Vapaavuori, O., Hielm-Bjorkman, A. and Jokinen, T. 2013. Topiramate as an add-on antiepileptic drug in treating refractory canine idiopathic epilepsy. J. Small Anim. Pract. 54, 512–520. Leppik, I.E. 1995. Felbamate. Epilepsia. 36, S66–S72. Leppik, I.E. and Wolff, D.E. 1995. The place of felbamate in the treatment of epilepsy. CNS Drugs. 4, 294–301. McGee, J.H., Erickson, D.J., Galbreath, C., Willigan, D.A. and Sofia, R.D. 1998. Acute, subchronic, and chronic toxicity studies with felbamate, 2-phenyl-1,3-propanediol dicarbamate. Toxicol. Sci. 45, 225–232. Munana, K. 2013. Management of refractory epilepsy. Top. Companion Anim. Med. 28, 67–71. Packer, M.A., Nye, G., Porter, S.E. and Volk, H.A. 2015. Assessment into the usage of levetiracetam in a canine epilepsy clinic. BMC Vet. Res. 11, 25; doi:10.1186/s12917-015-0340-x. Packer, R. and Volk, H. 2015. Epilepsy beyond seizures: a review of the impact of epilepsy and its comorbidities on health-related quality of life in dogs. Vet Rec. 177(12), 306–315. Platt, S. 2014. Felbamate. In Canine and feline epilepsy: diagnosis and management. Eds., De Risio, L. and Platt, S. Oxfordshire, UK: CABI, pp: 453–457. Reidenberg, P., Glue, P., Banfield, C.R., Colucci, R.D., Meehan, J.W., Radwanski, E., Mojavarian, P., Lin, C.C., Nezamis, J., Guillaime, M. and Affrime, M.B. 1995. Effects of felbamate on the pharmacokinetics of phenobarbital. Clin. Pharm. Therap. 58, 279–287. Ruehlmann, D., Podell, M. and March, P. 2001. Treatment of partial seizures and seizure-like activity with felbamate in six dogs. J. Small Anim. Pract. 42, 403–408. Ryvlin, P., Cucherat, M. and Rheims, S. 2011. Risk of sudden unexpected death in epilepsy patients given adjunctive antiepileptic treatment for refractory seizures: a meta-analysis of placebo-controlled randomized trials. Lancet Neurol. 10, 961–968. Thakkar, K., Billa, G., Rane, J., Chudasama, H., Goswami, S. and Shah, R. 2015. The rise and fall of felbamate as a treatment for partial epilepsy-aplastic anemia and hepatic failure to blame? Expert Rev. Neurother. 15, 1373–1375. Thomas, W.B. and Dewey, C.W. 2016. Seizures and narcolepsy. In Practical guide to canine and feline neurology. Eds., Dewey, C.W. and da Costa, R.C. 3rd ed. Ames, IA: Wiley, pp: 249–267. Volk, H. 2014. Pathophysiology of pharmacoresistant epilepsy. In Canine and feline epilepsy: diagnosis and management. Eds., De Risio, L. and Platt, S. Oxfordshire, UK: CABI, pp: 23–38. Wallis, R.A. and Panizzon, K.L. 1993. Glycine reversal of felbamate hypoxic protection. Neuroreport. 4, 951–954. Wasterlain, C.G., Adams, L.M., Hattori, H. and Schwartz, P.H. 1992. Felbamate reduces hypoxic brain damage in vivo. Eur. J. Pharmacol. 212, 275–278. Yang, J.T., Morris, M., Wong, K.K., Kucharczyk, N. and Sofia, R.D. 1992. Felbamate metabolism in pediatric and adult beagle dogs. Drug Metab. Disp. 20, 84–88. Zupanc, M.L., Werner, R.R., Schwabe, M.S., O’Connor, S.E., Marcuccilli, C.J., Hecox, K.E., Chico, M.S. and Eggener, K.A. 2010. Efficacy of felbamate in the treatment of intractable pediatric epilepsy. Pediatr. Neurol. 42, 396–403. | ||
| How to Cite this Article |
| Pubmed Style Dewey CW, Rishniw M, Sakovitch K. Felbamate as an oral add-on therapy in six dogs with presumptive idiopathic epilepsy and generalized seizures resistant to drug therapy. Open Vet. J.. 2022; 12(4): 445-450. doi:10.5455/OVJ.2022.v12.i4.5 Web Style Dewey CW, Rishniw M, Sakovitch K. Felbamate as an oral add-on therapy in six dogs with presumptive idiopathic epilepsy and generalized seizures resistant to drug therapy. https://www.openveterinaryjournal.com/?mno=99099 [Access: December 02, 2025]. doi:10.5455/OVJ.2022.v12.i4.5 AMA (American Medical Association) Style Dewey CW, Rishniw M, Sakovitch K. Felbamate as an oral add-on therapy in six dogs with presumptive idiopathic epilepsy and generalized seizures resistant to drug therapy. Open Vet. J.. 2022; 12(4): 445-450. doi:10.5455/OVJ.2022.v12.i4.5 Vancouver/ICMJE Style Dewey CW, Rishniw M, Sakovitch K. Felbamate as an oral add-on therapy in six dogs with presumptive idiopathic epilepsy and generalized seizures resistant to drug therapy. Open Vet. J.. (2022), [cited December 02, 2025]; 12(4): 445-450. doi:10.5455/OVJ.2022.v12.i4.5 Harvard Style Dewey, C. W., Rishniw, . M. & Sakovitch, . K. (2022) Felbamate as an oral add-on therapy in six dogs with presumptive idiopathic epilepsy and generalized seizures resistant to drug therapy. Open Vet. J., 12 (4), 445-450. doi:10.5455/OVJ.2022.v12.i4.5 Turabian Style Dewey, Curtis Wells, Mark Rishniw, and Kasie Sakovitch. 2022. Felbamate as an oral add-on therapy in six dogs with presumptive idiopathic epilepsy and generalized seizures resistant to drug therapy. Open Veterinary Journal, 12 (4), 445-450. doi:10.5455/OVJ.2022.v12.i4.5 Chicago Style Dewey, Curtis Wells, Mark Rishniw, and Kasie Sakovitch. "Felbamate as an oral add-on therapy in six dogs with presumptive idiopathic epilepsy and generalized seizures resistant to drug therapy." Open Veterinary Journal 12 (2022), 445-450. doi:10.5455/OVJ.2022.v12.i4.5 MLA (The Modern Language Association) Style Dewey, Curtis Wells, Mark Rishniw, and Kasie Sakovitch. "Felbamate as an oral add-on therapy in six dogs with presumptive idiopathic epilepsy and generalized seizures resistant to drug therapy." Open Veterinary Journal 12.4 (2022), 445-450. Print. doi:10.5455/OVJ.2022.v12.i4.5 APA (American Psychological Association) Style Dewey, C. W., Rishniw, . M. & Sakovitch, . K. (2022) Felbamate as an oral add-on therapy in six dogs with presumptive idiopathic epilepsy and generalized seizures resistant to drug therapy. Open Veterinary Journal, 12 (4), 445-450. doi:10.5455/OVJ.2022.v12.i4.5 |